
��ͼ��ʵ������ȡ��������ֳ�������װ�ã�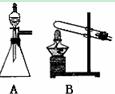
(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��
 ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
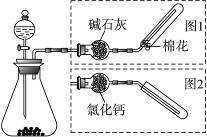
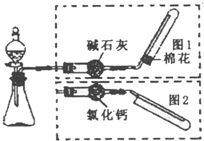 ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ���� ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ����
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ����
| ����װ���е�ҩƷ | ����ͼ���װ�� | |
| A | ��ʯ��ˮ | ͼ2 |
| B | ����ʯ��ϡ���� | ͼ1 |
| C | ͭ��ϡ���� | ͼ2 |
| D | �����ƺ�Ũ��ˮ | ͼ1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡ��������ֳ�������װ�ã�
(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������һ�и�һ12���¿���ѧ�Ծ� ���ͣ�ʵ����
��ͼ��ʵ������ȡ��������ֳ�������װ�ã�

(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com