���
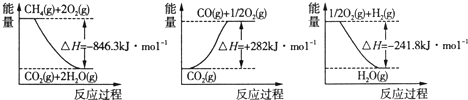
�⣺��1��������ͼ���â�CH
4��g��+2O
2��g����CO
2��g��+2H
2O��g����H=-846.3kJ?moL
-1��CO
2��g���TCO��g��+
O
2��g����H=+282kJ?moL
-1��
O
2��g��+H
2��g���TH
2O��g����H=-241.8kJ?moL
-1��-�ۡ�3+�ڵ�CH
4��g��+H
2O��g��
CO��g��+3H
2��g����H=��-846.3+241.8��3+282��kJ?moL
-1=+161.1kJ?moL
-1���ʴ�Ϊ��+161.1��
��2��ȼ�ϵ���У�������Ͷ��ȼ������Ͷ�ż���ĵ缫�Ǹ�����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��C
nH
2nO
n-4ne
-+nH
2O�TnCO
2+4nH
+��
�ʴ�Ϊ��C
nH
2nO
n-4ne
-+nH
2O�TnCO
2+4nH
+��
��3�����뷴Ӧ�������ڱ�״�������Ϊ448mL�����ʵ���Ϊ
=0.02mol�����ݵ���ת���غ��֪�����ɶ�����̼Ϊ
=0.01mol��n��NaOH��=0.1L��0.15mol?L
-1=0.015mol��n��NaOH����n��CO
2��=0.015mol��0.01mol=3��2����������2CO
2+3NaOH=Na
2CO
3+NaHCO
3+H
2O������������Һ�����ʵijɷּ����ʵ���֮��Ϊn��Na
2CO
3����n��NaHCO
3��=1��1����Һ��̼���ˮ�⣬̼�������ˮ����ڵ��룬��Һ�ʼ��ԣ���c��OH
-����c��H
+����̼�����ˮ��̶ȴ���̼���������c��HCO
3-����c��CO
32-����������Ũ�����ˮ��̶Ȳ���̼���Ũ��ԭ�������������ӣ���c��Na
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����
�ʴ�Ϊ��n��Na
2CO
3����n��NaHCO
3��=1��1��c��Na
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����
��4��a��ʱ��5CO��g��+I
2O
5��s��?5CO
2��g��+I
2��s��
��ʼ��/mol 4 0
ת����/mol x x
a����/mol 4-x x
����a��ʱCO
2����������գ�CO
2��=
=0.40����x=1.6mol
��ӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv��CO��=
=1.6mol?L
-1?min
-1���ʴ�Ϊ��1.6mol?L
-1?min
-1��
��5��T
1ʱ��5CO��g��+I
2O
5��s��?5CO
2��g��+I
2��s��
��ʼ��/mol 4 0
ת����/mol y y
b����/mol 4-y y
����b��ʱCO
2����������գ�CO
2��=
=0.80����y=3.2mol��c��CO��=0.4mol?L
-1��c��CO
2��=1.6mol?L
-1T
1ʱ��ѧƽ�ⳣ��K=
=
=1024��
�ʴ�Ϊ��1024��
��6��A����Ϊ����Ϊ���ݣ�����Ӧǰ�����������仯�����������������ܶȲ���ʱ��������Ӧ�ﵽƽ��״̬����A��ȷ��
B��c��Ϊ���㣬�������ʵ����ֱ���ȣ����������¶��£���ϵ�л�������ѹǿ���ȣ���B����
C����Ӧǰ������������䣬ѹǿ�仯��ƽ����Ӱ�죬CO��ת���ʲ��䣬��C��ȷ��
d��b���d��ʱ������CO
2���������˵�����еij̶ȴ���ѧƽ�ⳣ����K
b��K
d����D����
�ʴ�Ϊ��BD��





 ������A��B�ķ���ʽ����C2H4Br2��A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ
������A��B�ķ���ʽ����C2H4Br2��A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ

