
����Ŀ��Fe��Co��Ni��Ϊ�ڢ���Ԫ�أ����ǵĻ����������������������Ź㷺��Ӧ�á�
(1)��̬Coԭ�ӵļ۵����Ų�ʽΪ________��Co2������3d�ܼ�����________�ԳɶԵ��ӡ�
(2)Co3����һ��������[Co(N3)(NH3)5]2���У�Co3������λ����________��1 mol�������������Ҽ�����ĿΪ____________����λ��N3������ԭ�ӵ��ӻ�����Ϊ____________��
(3)Co2����ˮ��Һ����[Co(H2O)6]2�����ڡ���Co2������Һ�м��������ˮ�����ɸ��ȶ���[Co(NH3)6]2������ԭ����__________________________��
(4)ij��ɫ�����У�Fe2����Fe3���ֱ�ռ�������廥�����ڵĶ��㣬���������ÿ�����Ͼ���һ��CN����K��λ���������ijǡ��λ���ϡ��ݴ˿�֪�þ���Ļ�ѧʽΪ________����������Fe2�������������γɵĿռ乹����________��
(5)NiO�ľ����ṹ��ͼ����ʾ������ԭ���������AΪ(0,0,0)��BΪ(1,1,0)����Cԭ���������Ϊ________��
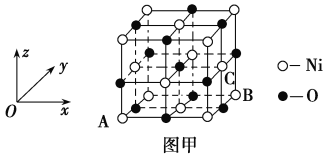
(6)һ���¶��£�NiO��������Է��ط�ɢ���γɡ������Ӳ㡱��������ΪO2��Ϊ���õ������У�Ni2���������(��ͼ��)����֪O2���İ뾶Ϊa pm��ÿƽ��������Ϸ�ɢ�ĸþ��������Ϊ_____g(�ú�a��NA�Ĵ���ʽ��ʾ)��
���𰸡�3d74s2 2 6 23NA sp NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2���γɵ���λ����ǿ KFe2(CN)6 ���������� (1��1/2��1/2) ![]()
��������
(1)��̬Coԭ�Ӻ�����27�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ�Coԭ��ʧȥ3����������Co3+��Co3+����3d�ܼ�����1�ԳɶԵ��ӣ�
(2)Co3+��һ��������[Co(N3)(NH3)5]2+�У�Co3+����λ����6��1����������������������ĿΪ6+2+3��5����λ��N3-����ԭ�Ӽ۲���ӶԸ���=2+![]() =2�����ݼ۲���ӶԻ��������жϸ������ӻ����ͣ�
=2�����ݼ۲���ӶԻ��������жϸ������ӻ����ͣ�
(3)NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ��������
(4)�þ�����Fe2+��Fe3+����ҵ���4��![]() =
=![]() ��CN-����=12��
��CN-����=12��![]() =3���������Ӿ���ʵ�����֪�������Ӹ���=
=3���������Ӿ���ʵ�����֪�������Ӹ���= =0.5����������Fe2+�����������γɵĿռ乹�����������壻
=0.5����������Fe2+�����������γɵĿռ乹�����������壻
(5)NiO�ľ���ṹ��ͼ����ʾ�����������������AΪ(0��0��0)��BΪ(1��1��0)��˵�������ⳤΪ1��C����λ�ھ������������ϣ�����X��Ϊ1������Y��Ϊ![]() ������Z��Ϊ
������Z��Ϊ![]() ��
��
(6)����ͼ֪��ÿ��Niԭ�ӱ�3��Oԭ�Ӱ�Χ��ÿ��Oԭ�ӱ�3��Niԭ�Ӱ�Χ����ͼ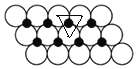 ��ʾ�����ڵ�3��Բ��������Ϊ�������Σ������εı߳�Ϊ2apm��ÿ�������κ���һ��Niԭ�ӣ������ε����=[
��ʾ�����ڵ�3��Բ��������Ϊ�������Σ������εı߳�Ϊ2apm��ÿ�������κ���һ��Niԭ�ӣ������ε����=[![]() ��2a��2a��sin60���10-24]m2=
��2a��2a��sin60���10-24]m2=![]() ��10-24a2m2����ͼ
��10-24a2m2����ͼ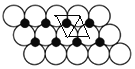 ʵ����ÿ��Niԭ�ӱ�����С�����ΰ���Сƽ���ı������Ϊ2
ʵ����ÿ��Niԭ�ӱ�����С�����ΰ���Сƽ���ı������Ϊ2![]() ��10-24a2m2��Oԭ�Ӹ���=
��10-24a2m2��Oԭ�Ӹ���=![]() ��6=1��ÿƽ��������Ϸ�ɢ�ĸþ��������=
��6=1��ÿƽ��������Ϸ�ɢ�ĸþ��������=![]() g��
g��
(1)��̬Coԭ�Ӻ�����27�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ�Coԭ��ʧȥ3����������Co3+�������۵����Ų�ʽΪ3d74s2��3d�ܼ��ϻ���Ҫ3������������Co3+����3d�ܼ�����2�Գɵ��ӣ�
(2)Co3+��һ��������[Co(N3)(NH3)5]2+�У�Co3+����λ����6��1����������������������ĿΪ6+2+3��5=23��1mol��������������������ĿΪ23NA����λ��N3-����ԭ�Ӽ۲���ӶԸ���=2+![]() =2�����ݼ۲���ӶԻ��������жϸ������ӻ�����Ϊsp��
=2�����ݼ۲���ӶԻ��������жϸ������ӻ�����Ϊsp��
(3)NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2+�γɵ���λ����ǿ��������Co2+����Һ�м��������ˮ�����ɸ��ȶ���[Co(NH3)6]2+��
(4)�þ�����Fe2+��Fe3+����ҵ���4��![]() =
=![]() ��CN-����=12��
��CN-����=12��![]() =3���������Ӿ���ʵ�����֪�������Ӹ���=
=3���������Ӿ���ʵ�����֪�������Ӹ���=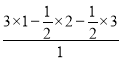 =0.5����þ����м����ӡ�Feԭ�ӡ�CN-����֮��=0.5��(0.5��2)��3=1��2��6���仯ѧʽΪKFe2(CN)6����������Fe2+�����������γɵĿռ乹�����������壻
=0.5����þ����м����ӡ�Feԭ�ӡ�CN-����֮��=0.5��(0.5��2)��3=1��2��6���仯ѧʽΪKFe2(CN)6����������Fe2+�����������γɵĿռ乹�����������壻
(5)NiO�ľ���ṹ��ͼ����ʾ�����������������AΪ(0��0��0)��BΪ(1��1��0)��˵�������ⳤΪ1��C����λ�ھ������������ϣ�����X��Ϊ1������Y��Ϊ![]() ������Z��Ϊ
������Z��Ϊ![]() ������C�����Ϊ(1��
������C�����Ϊ(1��![]() ��
��![]() )��
)��
(6)����ͼ֪��ÿ��Niԭ�ӱ�3��Oԭ�Ӱ�Χ��ÿ��Oԭ�ӱ�3��Niԭ�Ӱ�Χ����ͼ ��ʾ�����ڵ�3��Բ��������Ϊ�������Σ������εı߳�Ϊ2apm��ÿ�������κ���һ��Niԭ�ӣ������ε����=[
��ʾ�����ڵ�3��Բ��������Ϊ�������Σ������εı߳�Ϊ2apm��ÿ�������κ���һ��Niԭ�ӣ������ε����=[![]() ��2a��2a��sin60���10-24]m2=
��2a��2a��sin60���10-24]m2=![]() ��10-24a2m2����ͼ
��10-24a2m2����ͼ ʵ����ÿ��Niԭ�ӱ�����С�����ΰ���Сƽ���ı������Ϊ2
ʵ����ÿ��Niԭ�ӱ�����С�����ΰ���Сƽ���ı������Ϊ2![]() ��10-24a2m2��Oԭ�Ӹ���=
��10-24a2m2��Oԭ�Ӹ���=![]() ��6=1��ÿƽ��������Ϸ�ɢ�ĸþ��������=
��6=1��ÿƽ��������Ϸ�ɢ�ĸþ��������=![]() g=
g=![]() g=
g=![]() g��
g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
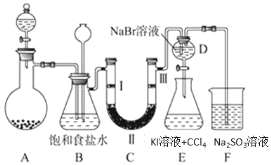
��1��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C���������������η�______
a | b | c | d | |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | ��ˮ�Ȼ��� | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��2�����װ��D��E��Ŀ���DZȽ��ȡ��塢�ⵥ�ʵ�������ǿ��������D�л���ͨ��һ����������ʱ�����Կ�����ɫ��Һ��Ϊ�Ȼ�ɫ����������װ��D��������Һ����װ��E�У����۲쵽���������²���Һ����ɫ����֤�����嵥�ʵ�������ǿ�ڵⵥ�ʣ�������ͬѧ�Ըý���������飬����������___________��
��3���ձ�F�е� ����������Һ��������β�������ʵ�鷽����֤β�����պ���Һ�к��� SO42-_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й���ѧԺ�����Ŷ��о��������ڳ��³�ѹ�Ϳɼ����£�����![]() ��һ�ֹ���������ϳ�
��һ�ֹ���������ϳ�![]() ��ԭ��ʾ��ͼ���¡��Իش��������⣺
��ԭ��ʾ��ͼ���¡��Իش��������⣺
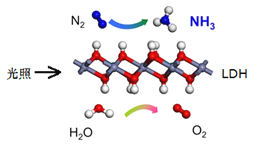
��1��������ת���ĽǶȣ��ù��̽�______ת����Ϊ__________��
��2���÷�Ӧ�Ļ�ѧ����ʽΪ��________________���������뻹ԭ�������ʵ���֮��Ϊ__________��
��3�����ڸ÷�Ӧ��˵������ȷ����_______
A������������ѧ��Ӧ���ʣ����̷�Ӧʱ�䣬�Ӷ���߲���Ч��
B���÷�Ӧ�������漰���Լ��ͷǼ��Լ��Ķ��Ѻ��γ�
C������ͨ������Һ̬�����ķ�����õ���
D������![]() �ϳ�
�ϳ�![]() �Ĺ��������˹��̵�
�Ĺ��������˹��̵�
��4���ִ���ҵ�����Ȼ��ơ�������̼�Ͱ���Ϊԭ���Ʊ��������ԭ�ϵ�˳���ǣ����Ȼ�����Һ��ͨ��_________�����ͣ���ͨ��������_________���Ƶ�![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ұ�����������Ϊ98%,�ܶ�Ϊ1.84 g��cm-3������,�ݴ�����˵��������� (����)
A. ����������ʵ���Ũ��Ϊ18.4 mol��L-1
B. ������50 mL��������ͭ��Ӧ�ɵõ���״����SO2 0.46 mol
C. ijͬѧ�ø���������ϡ����ʱ,δϴ���ձ��Ͳ�����,������������Ƶ�ϡ����Ũ��ƫ��
D. ��������ˮ���������������Һ�����ʵ���Ũ��С��9.2 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ����������������
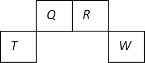
A. T������������Ӧ
B. Ԫ�صķǽ����ԣ�Qǿ��W
C. W�ĵ��ʹ���ʱ������������������ˮ����Ũ��Һ��Ӧ
D. ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���Ƕ���������Ԫ�أ���ԭ��������������A��Bͬ���ڣ�A��Dͬ���壬Aԭ���������������ڲ�������Ķ�����BԪ������������������������B����������C�������ӵ��Ӳ�ṹ��ͬ��C�ĵ�����B�ĵ����ڲ�ͬ�����·�Ӧ��������C2B��C2B2,E������������ԭ�Ӱ뾶��С��Ԫ�أ���ش�:
��1��D��Ԫ�����ڱ��е�λ����___________��
��2��C2B2�ĵ���ʽ��________��
��3��B��C��E�����Ӱ뾶�ɴ�С��˳��Ϊ________(�����ӷ��Żش�)��A��D��EԪ������������Ӧˮ�����������ǿ����Ϊ_________(�û�ѧʽ�ش���ͬ),B����̬�⻯����H2S��ȷе�ߵ���_____��ԭ����___________________________��
��4��д��D��������������ᷴӦ�Ļ�ѧ����ʽ____________________________________��
��5��1mol C2B2��������ˮ��Ӧת�Ƶ��ӵ����ʵ�����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ж�ͼ���������ȷ����
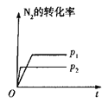
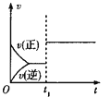
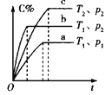

�� �� �� ��
A.ͼ�ɱ�ʾѹǿ�Է�Ӧ��![]()
![]() ��Ӱ��
��Ӱ��
B.ͼ���У�![]() ʱ�̸ı������һ���Ǽ����˴���
ʱ�̸ı������һ���Ǽ����˴���
C.��ͼ����ʾ��Ӧ��![]() ����
����![]() ��
��![]()
D.ͼ����ʾˮ��![]() ��
��![]() �Ĺ�ϵ��ABC������������
�Ĺ�ϵ��ABC������������![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��![]() +CH3CHO+HBr
+CH3CHO+HBr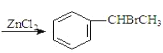 +H2O��±�������Ӧ��;
+H2O��±�������Ӧ��;
![]() +
+![]()
![]()
![]() +NaX
+NaX
�ñ�Ϊԭ�Ϻϳɻ����������·���£�
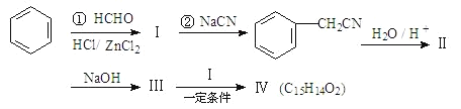
���У��������ᡣ��ش��������⣺
(1)�ڵķ�Ӧ������________��
(2)д��ͬʱ�������������Ļ�������ͬ���칹��ṹ��ʽ(д2��)_____��_____��
a.����FeCl3��Һ��������ɫ��
b.�ܷ���������Ӧ��
c.�˴Ź������׳��������������壬�����֮��ΪΪ 1��2��1��
(3)1mol���������ȫȼ������O2_____mol����������Ľṹ��ʽ��__________��
(4)����������Ҵ���Ũ���Ṳ�ȣ��ϳ�һ���㾫ԭ�ϣ���д���÷�Ӧ�Ļ�ѧ����ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijԪ�ص�һ��ԭ���γɵ����ӿɱ�ʾΪ![]() n-������˵����ȷ���ǣ� ��
n-������˵����ȷ���ǣ� ��
A.![]() n-�к��е�������Ϊa+b
n-�к��е�������Ϊa+b
B.![]() n-�к��еĵ�����Ϊa-n
n-�к��еĵ�����Ϊa-n
C.Xԭ�ӵ�������Ϊa+b+n
D.һ��Xԭ�ӵ�����ԼΪ![]() g
g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com