
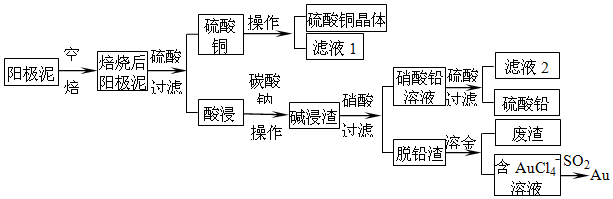
���� ��ʵ�����̿�֪����ͭ������������ຬ��Cu��Au���𣩺�PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ�ӣ�����������ͭ���壻�����Ĺ����к���Au���𣩺�PbSO4�����ʾ�̼���ƽ�ϴ������Ǧת����̼��Ǧ���ü����ΪAu��PbCO3��Ũ�����������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ𣬼���ˮ�ܽ𣬵õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au��
��1����ͭ�е�ͭ��Ǧ�ᷢ��ʧ���ӵ�������Ӧ��
��2��������ijɷ�ΪCu��Au���𣩺�PbSO4 �ȣ��ڱ��չ�����ͭ������ΪCuO�����������ֵķ��飬����Ӵ����������߱���Ч�ʣ�
��3������I�IJ����Ǵ�����ͭ��Һ�л������ͭ���壻
��4��SO2��ԭAuCl4-��Ӧ�õ�����������SO42-����ԭ������Au����ƽ��д����ʽ��
��5������Һ1��������ͭ��Һ����ѭ�������������ڳ�����ã�
��6����1mol PbSO4�������1L Na2CO3��Һ�У���Һ��c��SO42-��=1mol/L������Ksp��PbCO3��=c��CO32-����c��Pb2+����c��CO32-���ɼ������Һ�е�c��Pb2+����������c��Pb2+����Ksp��PbSO4��=c��SO42-����c��Pb2+�������������Һ��c��SO42-�������������Ϣ�жϣ�
��� �⣺��ʵ�����̿�֪����ͭ������������ຬ��Cu��Au���𣩺�PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ�ӣ�����������ͭ���壻�����Ĺ����к���Au���𣩺�PbSO4�����ʾ�̼���ƽ�ϴ������Ǧת����̼��Ǧ���ü����ΪAu��PbCO3��Ũ�����������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ𣬼���ˮ�ܽ𣬵õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au��
��1����⾫���Ĵ�ͭ�������ᷢ��������Ӧ�����е���ͭ�ͻ����Ա�Cuǿ�Ľ������ᷢ���ܽ⣬��˴�ͭ�е�ͭ��Ǧ�ᷢ��ʧ���ӵ�������Ӧ���缫��ӦʽΪCu-2e-=Cu2+��Pb-2e-+SO42-=PbSO4��
�ʴ�Ϊ��Cu-2e-=Cu2+��
��2�������Ĺ����к���Au���𣩺�PbSO4�����ʾ�̼���ƽ�ϴ������Ǧת����̼��Ǧ���ü����ΪAu��PbCO3��
�ʴ�Ϊ��Au��PbCO3��
��3������I�IJ����Ǵ�����ͭ��Һ�л������ͭ���壬��˸ò���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��裬�ʴ�Ϊ������Ũ�������½ᾧ�����ˣ�
��4��SO2��ԭAuCl4-�л�ԭ�������������Ƚ���ȷ����˺������Ʋ������������SO42-����ԭ������Au����������ԭ��Ӧ��ʧ�����غ���ȱ����ƽ��Ȼ����ݵ���غ�����ƽ����õ��ķ�Ӧ����ʽΪ2AuCl4-+3SO2+6H2O=2Au+3SO42-+8Cl-+12H+��
�ʴ�Ϊ��2AuCl4-+3SO2+6H2O=2Au+3SO42-+8Cl-+12H+��
��5����Һ1���ڽᾧ����ͭʱʣ�µ���Һ��������������δ����������ͭ����˲���ǰ�������ͭ��Һ����ѭ���������ڳ�����ã�
�ʴ�Ϊ������Һ1����CuSO4��Һ�У�
��6����1mol PbSO4�������1L Na2CO3��Һ�У���Һ��c��SO42-��=1mol/L������Ksp��PbCO3��=c��CO32-����c��Pb2+��=1.5��10-13��c��CO32-��=5 mol/L��֪����Һ�е�c��Pb2+��=3��10-14mol/L���ٸ���c��Pb2+����Ksp��PbSO4��=c��SO42-����c��Pb2+����֪��������Һ��c��SO42-��=$\frac{1.8��1{0}^{-8}}{3��1{0}^{-14}}$=6��105mol/L��1mol/L��������Һ�е����������û�г���������c��SO42-��=1mol/L��
�ʴ�Ϊ��1mol/L��
���� ���⿼����������ᴿ��Ϊ��Ƶ���㣬�漰���ʵķ��롢���ӵļ��顢���ԭ����������ת����֪ʶ������ʵ�����̼����ʵ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
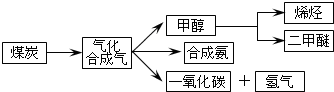
| ���� | CH3OH | CH3OCH3 | H2O |
| c/��mol•L-1�� | 0.8 | 1.24 | 1.24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
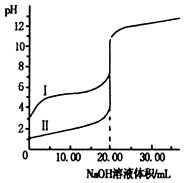 �����£���0.1mol•L-1 NaOH��Һ�ֱ�ζ�20.00ml0.1mol•L-1������ʹ�����Һ���ζ�������ͼ������˵����ȷ���ǣ�������
�����£���0.1mol•L-1 NaOH��Һ�ֱ�ζ�20.00ml0.1mol•L-1������ʹ�����Һ���ζ�������ͼ������˵����ȷ���ǣ�������| A�� | I����ֱ��ʾ����ʹ���ĵζ����� | |
| B�� | V��NaOH��=10.00mLʱ��$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$��1 | |
| C�� | pH=7ʱ��������������NaOH��Һ�������� | |
| D�� | V��NaOH��=20.00mLʱ��c��Clһ����c��CH3COOһ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ����������ԭ��Ӧ | B�� | ����ֻ���������� | ||
| C�� | N2O4�ǻ�ԭ�� | D�� | N2O4�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
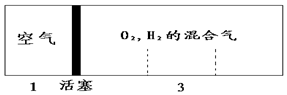 �ܱ������������ƶ��Ļ������߷ֱ���������H2��O2�Ļ�����壬��ʼ�����Ϊ1��3������H2��O2�Ļ�������ȼ�������ָ���ԭ���¶ȣ�ˮΪҺ̬���������һ�ͣ���������������룬��ԭ��H2��O2������ȿ���Ϊ��������
�ܱ������������ƶ��Ļ������߷ֱ���������H2��O2�Ļ�����壬��ʼ�����Ϊ1��3������H2��O2�Ļ�������ȼ�������ָ���ԭ���¶ȣ�ˮΪҺ̬���������һ�ͣ���������������룬��ԭ��H2��O2������ȿ���Ϊ��������| A�� | 1��2 | B�� | 3��2 | C�� | 5��2 | D�� | 7��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com