�⣺��1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ���������˽���������Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ϊ�˳�ȥMg
2+�����������BaCl
2��Һ��Ϊ�˳�ȥSO
42-�����������Na
2CO
3��Һ��Ϊ�˳�ȥ������Ca
2+�Ͷ����Ba
2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO
32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壮���������˳���Ҫ����Na
2CO
3��Һ������BaCl
2��Һ֮����룬���������ᣬ����̼��Ƶ��ܶȻ��������㣬ҪʹCa
2+��ȫ������Ӧ��
c��Ca
2+����c��CO
2-3����2.9��10
-9����c��CO
2-3����
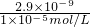
=2.9��10
-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10
-4mol/L��
��2��ʯ��ʯ�������ɵ�����MΪCO
2�����������պ�IJ�����Ҫ��SiO
2��Al
2O
3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO
2��Na
2SiO
3������ͨ������CO
2������Ӧ�ķ���ʽΪ��NaAlO
2+CO
2+2H
2O=Al��OH��
3��+NaHCO
3��Na
2SiO
3+2CO
2+2H
2O=H
2SiO
3+2NaHCO
3�����ӷ���ʽΪ��SiO
32-+2CO
2+2H
2O�TH
2SiO
3��+2HCO
3-��AlO
2-+2H
2O+CO
2�TAl��OH��
3��+HCO
3-��
�ʴ�Ϊ��SiO
32-+2CO
2+2H
2O�TH
2SiO
3��+2HCO
3-��AlO
2-+2H
2O+CO
2�TAl��OH��
3��+HCO
3-��
��3��̼����ˮ��ʼ��ԣ�����CO
32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬���У�c��Na
+����c��CO
32-����c��OH
-����c��HCO
3-����c��H
+����
�ʴ�Ϊ��c��Na
+����c��CO
32-����c��OH
-����c��HCO
3-����c��H
+����
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã��ʴ�Ϊ��CO
2��
��5�������������������غ���㣬�����ɷ���ɸ������Ϊx������1t��35%=28%��xt��x=
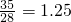
t��
�ʴ�Ϊ��1.25��
��������1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ����ע��һ�㣬�Ǿ�����������������Na
2CO
3��Һ��Ϊ�˳�ȥ������Ca
2+�Ͷ����Ba
2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO
32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壻����̼��Ƶ��ܶȻ��������㣻
��2��ʯ��ʯ�������ɵ�����MΪCO
2�����������պ�IJ�����Ҫ��SiO
2��Al
2O
3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO
2��Na
2SiO
3���Դ���д���ӷ���ʽ��
��3��̼����ˮ��ʼ��ԣ�����CO
32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬�Դ��ж�����Ũ�ȴ�С˳��
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã�
��5�������������������غ���㣮
������������һ���ԡ�����ɸ�����Ʊ�Ϊ�����Ļ�ѧ���������⣬����ȡ�Ľ��£�����������ǻ�ѧ����֪ʶ�������������ʵ�ʣ��Խ����ѧʵ��������˼·�������ʣ�ʹ�����龳��ʵ��������ֻ�ѧ��STSE�Ĺ�ϵ������ѧ�Ա�ɫ��������ѧ��������ϵʵ�ʣ�ѧ�����õ�ѧϰ�ۣ����⿼���֪ʶ��࣬Ҳ�dz���˼ά�����ϴ���Ŀ�Ի�ѧ֪ʶ�������Ӧ��֪ʶ������������������й������Ǩ����������Ҫ��ϸߣ�������ӱ���Ķ������ܿ���ѧ�����Ķ����������ϵ��ռ�����������ͬʱ��ѧ���������������������⡢������⡢���ֱ���ȷ����������Ҫ��dz��ߣ���һ���ۺ������⣬��ѧ���Ժ��ѧϰ���������õ�ָ�����ã�


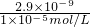 =2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L��
=2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L��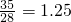 t��
t��


