
����ʵ������������ȷ���� �� ��
A��������Һ�IJ����У�ת����Һ���������ձ�δϴ�ӻ�ʹ��������ҺŨ��ƫ��
B��ϴ�ӳ����IJ����ǽ��������ڹ������У����ò��������������ˮ��ϴ
C��ʵ��������950 mL 0��2 mol/L��CuSO4��Һʱ�����ȡ����������Ϊ50��0 g
D���Ʊ�Fe��OH��3����ʱ�������͵�FeCl3��Һ�����ˮ�У����ȱ߽��裬ֱ���õ����ɫ��Һ
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
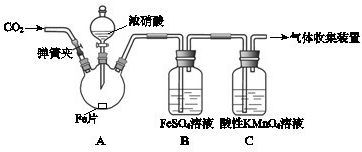
| ʵ����� | ʵ������ |
| ���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼУ� | |
| ��Һ©����������Ũ���Ỻ��������ƿ�У��رջ����� | ���������� |
| ������ƿ����Ӧ��ʼ��ֹͣ���ȣ� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz�� B����Һ����ɫ�� C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�࣮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���������� | ���ͻ���� |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� | �������ۻ��������������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� | ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3 ���� |
��2mL 1mol/L NaOH��Һ���ȼ���3��1mol/L MgCl2��Һ���ټ���3��1mol/L FeCl3��Һ |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� | ����Һ��һ�����д�����SO42- |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3���� |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������и�����ҵ��������һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������������Ӧԭ���Ľ��͡����۾���ȷ��һ����
���������� ���ͻ����
A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� �������ۻ���������������
B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ�
C���������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ�����ȣ�δ����ɫ���� ����δˮ��
D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� ����Һ��һ�����д�����SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijѧϰ��ȤС��̽���ϸɵ�أ�пͲ������̿�ۡ�MnO2��NH4Cl�ȵĺ�״��Ļ������ã����û��յ����ʽ�������ʵ�顣
I����1���ӷϸɵ������ȡNH4Cl��
�� ���øú�״����ȡNH4Clǰ�����IJ���Ϊ��a���ܽ� b�� ��
�� ��ͬѧ���룺���������NH4Cl��Һ�������ᾧ�����գ��Ϳ����Ƶô�����NH4Cl����Լ�ͬѧ�ķ����������۲�˵�����ɣ�______________________________________
��
��2����ȡ������
�� ��ͬѧҪ�Ʊ����ռ��������İ��������и���Ӧ�������к�������
a�����Ȼ�粒�����ȷֽ� b����Ũ��ˮ�����������ƹ�����
c�����������ƹ������Ũ��ˮ�� d�����Ȼ��Ũ��Һ�����������ƹ�����
�� ��ͬѧ��Ϊ������ƿ���������ϣ���ͼ��ʾ�����Ϳ����ռ��������İ�����
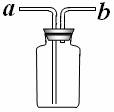
������Ϊ���У���˵���������_____________________________��
������Ϊ�����У���˵�������ɣ�___________________________��
��ͬѧ��������õ��İ�����ȡ��ˮ������������̽��ʵ�飺
��3��Ϊ̽��NH3?H2O�Ƿ���������ʡ�
��ͬѧ��Ʒ������£��� ��1.12L�������NH3��ȫ����ˮ�������Һ500mL��
�� ���۷������ݣ���
�ɵó����ۡ�
������ʵ�鲽��ڣ���д������Ŀո��С�
��4��̽����ˮ������ķ�Ӧ���̡�
��ͬѧ����������ʵ�飺��25mL������ˮ����εμ�ͬŨ�ȵ����ᣬ�ⶨ��Ӧ��������ҺpH��������pH�仯���ߣ���ͼ������ش�
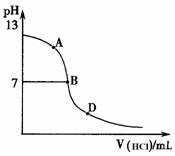
�����ǡ����ȫ�к�ʱ��pH��Ӧ��_________
����A��B��D������ʱ��Һ��c(NH3?H2O)��c(NH4+)��______mol?L��1����Һ��
�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
III�������̽��
��5�����������̽��ʵ�鱨�档
��̽�����⡿�Ƚ���25�桢0.1mol?L-1��NH3?H2O��Һ��0.1mol?L-1��NH4Cl��Һ�У�NH3?H2O�ĵ���̶���NH4+ˮ��̶ȵ���Դ�С��
��̽��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com