
�����еIJ���Դ��O3�Ժ�ˮ��I������������O3����ͨ��NaI��Һ�н���ģ���о���
(1)O3��I��������I2�Ĺ�����3����Ӧ��ɣ�
��I��(aq)��O3(g)===IO��(aq)��O2(g)����H1��
��IO��(aq)��H��(aq)  HOI(aq)����H2��
HOI(aq)����H2��
��HOI(aq)��I��(aq)��H��(aq)  I2(aq)��H2O(l)����H3��
I2(aq)��H2O(l)����H3��
�ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________________________________________���䷴Ӧ�Ȧ�H��________��
(2)����Һ�д��ڻ�ѧƽ�⣺I2(aq)��I��(aq)  I
I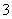 (aq)����ƽ�ⳣ������ʽΪ______________��
(aq)����ƽ�ⳣ������ʽΪ______________��
(3)Ϊ̽��Fe2����O3����I����Ӧ��Ӱ��(��Ӧ��ϵ��ͼ0)��ij�о�С��ⶨ����ʵ����I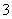 Ũ�Ⱥ���ϵpH�������ͼ1���±���
Ũ�Ⱥ���ϵpH�������ͼ1���±���
| ��� | ��Ӧ�� | ��ӦǰpH | ��Ӧ��pH |
| ��1�� | O3��I�� | 5.2 | 11.0 |
| ��2�� | O3��I����Fe2�� | 5.2 | 4.1 |
�ٵ�1��ʵ���У����·�Ӧ��pH���ߵ�ԭ����__________________________________
________________________________________________________________________��
��ͼ�е�AΪ________����Fe3������A�Ĺ������������I����ת���ʣ�ԭ����
________________________________________________________________________
________________________________________________________________________��
�۵�2��ʵ�����18 s��I Ũ���½��������½���ֱ��ԭ����(˫ѡ)________��
Ũ���½��������½���ֱ��ԭ����(˫ѡ)________��
A��c(H��)��С��������B��c(I��)��С
C��I2(g)�������� D��c(Fe3��)����
(4)��ͼ1������3��18 s�ڵ�2��ʵ��������I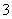 ��ƽ����Ӧ����(д��������̣����������λ��Ч����)��
��ƽ����Ӧ����(д��������̣����������λ��Ч����)��
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���Ԫ�����ڱ���һ���֣������ѱ���ˢ�-�����Ԫ�ء�
| ���� |
| |||||||||||||||||
| 1 | ||||||||||||||||||
| 2 | �� | �� | ||||||||||||||||
| 3 | �� | �� | �� | �� | �� | |||||||||||||
| 4 | �� | �� | ||||||||||||||||
���������Ԫ�ػش��������⣺
��1�����ڹ���Ԫ�ص��� ���ñ������ֱ�ʾ����
��2����������ǿ��Ԫ���� ����Ԫ�ط��ţ���
��3��Ԫ�آ��γɵ���ʹƷ����Һ��ɫ����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��4��д��Ԫ�آۺ�Ԫ�آ��γɵĻ�����ĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ��Һ���ܴ������棬�Ҽ������ϡ����ʱ�����������ɵ��ǣ� ��
A��Na+��Ag+��CO32����Cl�� B��K+��Ba2+��SO42����Cl��
C��Na+��K+��CO32����Cl�� D��H+��K+��Cl����HCO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��4 mol A�����2 mol B������2 L�������л�ϣ�����һ�������·������·�Ӧ��2A(g)��B(g)  2C(g)������2 s����C��Ũ��Ϊ0.6 mol·L��1���������м���˵����
2C(g)������2 s����C��Ũ��Ϊ0.6 mol·L��1���������м���˵����
��������A��ʾ��Ӧ��ƽ������Ϊ0.3 mol·L��1·s��1
��������B��ʾ��Ӧ��ƽ������Ϊ0.6mol·L��1·s��1
��2 sʱ����A��ת����Ϊ70%
��2 sʱ����B��Ũ��Ϊ0.7 mol·L��1
������ȷ���� (����)
A���٢� B���٢� C���ڢ� D���ۢ�
(2)���٢�����A��B��ʾ��Ӧ��ƽ�����ʷֱ�Ϊ0.3 mol·L��1·s��1��0.6 mol·L��1·s��1���������ʱ�ʾ�Ļ�ѧ��Ӧ���ʸ��죿
(3)������A��ת���ʾ�������Ϊ30%����ô������B��ת����Ϊ���٣�����Ѹ�ٵó�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ػ�ѧ֪ʶ�����жϣ����н��۴������(����)
A��ij���ȷ�Ӧ���Է����У���˸÷�Ӧ��������Ӧ
B��NH4Fˮ��Һ�к���HF�����NH4F��Һ���ܴ���ڲ����Լ�ƿ��
C����ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں���
D������Ӧ��Ũ�ȿɼӿ� ��Ӧ���ʣ������Ũ����������Ӧ����������H2������
��Ӧ���ʣ������Ũ����������Ӧ����������H2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʹ��Ӧ4NH3(g)��3O2(g)===2N2(g)��6H2O(g)��2 L���ܱ������н��У�����Ӻ�N2�����ʵ���������0.6 mol���˷�Ӧ��ƽ������v( X)Ϊ (����)
X)Ϊ (����)
A��v(NH3)��0.02 mol·L��1·s��1
B��v(O2)��0.01 mol·L��1·s��1
C��v(N2)��0.02 mol·L��1·s��1
D��v(H2O)��0.02 mol·L��1·s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����0.1 mol��MnO2��ĩ��50 mL�����������Һ��(�ܶ�Ϊ1.1 g·mL��1)���ڱ�״���·ų�����������ʱ��Ĺ�ϵ����ͼ��ʾ���ش��������⣺
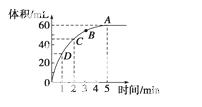
(1)A��B��C��D�ĵ㻯ѧ��Ӧ���ʿ�����˳��Ϊ
________________________________________________________________________��
(2)���ͷ�Ӧ���ʱ仯��ԭ��
________________________________________________________________________��
(3)�����������ij�ʼ ���ʵ���Ũ��
���ʵ���Ũ��
________________________________________________________________________��
(4)��Ӧ���е�2����ʱ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܹ�ʹ��ӦCu+2H2O====Cu(OH)2+H2����������( )
A����ͭƬ����������������Ȼ�ͭ��Һ
B����ͭƬ����������������������Һ
C��ͭп�Ͻ��ڳ�ʪ�����з����绯ѧ��ʴ
D��ͭƬ��ԭ��صĸ�����̼����ԭ��ص��������Ȼ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����ƺ��ƵĻ��������������ȷ����( )
A. ���ȶ��ԣ�Na2CO3>NaHCO3
B��Na2O��Na2O2���ܺ�ˮ��Ӧ���ɼ���Ƕ��Ǽ���������
C��̼�����ƿ���������θ�����
D��Na2O2�����������ӵĸ�����Ϊ1�� 2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com