
| A�� | ��c��CH3COOH��=0.1mol/L����Ӧ�����Һ�У�c��CH3COO-��+c��OH-��=c��Na+��+c��H+�� | |
| B�� | ��c��CH3COOH��=0.1mol/L����Ӧ�����Һ�У�c��OH-����c��CH3COOH��+c��H+�� | |
| C�� | ��c��CH3COOH��=0.2mol/L����Ӧ�����Һ�У�c��CH3COO-����c��Na+����c��CH3COOH����c��H+�� | |
| D�� | ����Ӧ�����Һ�����ԣ���c��CH3COOH��+c��CH3COO-����0.05mol/L |
���� A����c��CH3COOH��=0.1mol/L����������ȡ����ʵ���Ũ����ȣ��������ʵ�����ȣ���Ϻ����ǡ�÷�Ӧ���ɴ����ƣ��κε������Һ�����ڵ���غ㣬���ݵ���غ��жϣ�
B����c��CH3COOH��=0.1mol/L����������ȡ����ʵ���Ũ����ȣ��������ʵ�����ȣ���Ϻ����ǡ�÷�Ӧ���ɴ����ƣ��κε������Һ�ж����������غ㣬���������غ��жϣ�
C����c��CH3COOH��=0.2mol/L���������Ϻ���Һ�е�����Ϊ�����ʵ���Ũ�ȵ�CH3COOH��CH3COONa���������̶ȴ��ڴ��������ˮ��̶ȣ���Һ�����ԣ�
D�������Ƴʼ��ԣ�Ҫʹ�����Һ�����ԣ������Ӧ����������
��� �⣺A����c��CH3COOH��=0.1mol/L����������ȡ����ʵ���Ũ����ȣ��������ʵ�����ȣ���Ϻ����ǡ�÷�Ӧ���ɴ����ƣ��κε������Һ�����ڵ���غ㣬���ݵ���غ��c��CH3COO-��+c��OH-��=c��Na+��+c��H+������A��ȷ��
B����c��CH3COOH��=0.1mol/L����������ȡ����ʵ���Ũ����ȣ��������ʵ�����ȣ���Ϻ����ǡ�÷�Ӧ���ɴ����ƣ��κε������Һ�ж����������غ㣬���������غ��c��OH-��=c��CH3COOH��+c��H+������B����
C����c��CH3COOH��=0.2mol/L���������Ϻ���Һ�е�����Ϊ�����ʵ���Ũ�ȵ�CH3COOH��CH3COONa���������̶ȴ��ڴ��������ˮ��̶ȣ���Һ�����ԣ�����ĵ���̶Ƚ�С��������Һ��c��CH3COO-����c��Na+����c��CH3COOH����c��H+������C��ȷ��
D�������Ƴʼ��ԣ�Ҫʹ�����Һ�����ԣ������Ӧ������������Ϻ���Һ������������ʵ���Ũ�ȼ�С����������غ��c��CH3COOH��+c��CH3COO-����0.05mol/L����D��ȷ��
��ѡB��
���� �������������Һ�����ж�Ϊ���忼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬��ȷ��Һ�����ʼ��������ǽⱾ��ؼ���ע�����غ�������غ��������ã��״�ѡ����D��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥCO2�л��е�����HCl���壬�ɽ���ͨ������Na2CO3��Һ | |
| B�� | �����л����������ۣ��ɼ���NaOH��Һ����ַ�Ӧ����˳�ȥ | |
| C�� | ��ij�����������ɫʵ�飬�۲���ɫΪ��ɫ��˵������K+ | |
| D�� | ��ij��Һ�м���BaCl2��Һ������������ɫ������˵����Һ�к���SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COONa����Һ��CH3COO-+H2O�TCH3COOH+OH- | |
| B�� | NH4Cl����Һ��NH4++H2O?NH3•H2O+H+ | |
| C�� | Na2CO3����Һ��CO32-+H2O?H2CO3+2OH- | |
| D�� | AlCl3����Һ��Al3++3H2O�TAl��OH��3��+3H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��1��FeCl3���о�ˮ���ã�����ʴ�豸��FeCl3��Һ��ʴ�����豸����H+�����⣬��һ��Ҫԭ���ǣ������ӷ���ʽ��ʾ��2Fe3++Fe=3Fe2+��
��1��FeCl3���о�ˮ���ã�����ʴ�豸��FeCl3��Һ��ʴ�����豸����H+�����⣬��һ��Ҫԭ���ǣ������ӷ���ʽ��ʾ��2Fe3++Fe=3Fe2+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 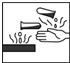 | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������c��NH4+����ͬ��������Һ����NH4Al��SO4��2��NH4Cl��CH3COONH4�����ʵ���Ũ���ɴ�С��˳��Ϊ�ڣ��٣��� | |
| B�� | 0.001mol•L-1��ˮ�У�c��Cl2��+c��Cl-��+c��ClO-��+c��HClO��=0.01mol•L-1 | |
| C�� | amol•L-1�Ĵ�����0.01mol•L-1������������Һ�������Ϻ�����ԣ�������Ka=5��10-10/��a/2-0.005�� | |
| D�� | ��5mL��KCl��KlŨ�Ⱦ�Ϊ0.01mol•L-1�Ļ����Һ�У��μ�8mL0.01mol•L-1��AgNO3��Һ��������Һ������Ũ�ȴ�С��ϵΪc��NO3-����c��K+����c��Cl-����c��I-����c��Ag+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��S��0����H��0 | B�� | ��S��0����H��0 | C�� | ��S��0����H��0 | D�� | ��S��0����H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
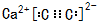 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | �� | �� | �� | �� |
| M����ĩ��Ʒ��/g | 0.90 | 1.80 | 3.60 | 7.20 |
| M����Ӧ��ʣ����壩/g | 0 | 0.64 | 2.48 | 6.08 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com