
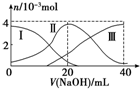
 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯�����ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ���V��NaOH��=20mLʱ����Һ�и�����Ũ�ȵĴ�С˳����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯�����ͼ��ʾ�����Т����H2A�������HA-�������A2-��������ͼʾ�жϣ���V��NaOH��=20mLʱ����Һ�и�����Ũ�ȵĴ�С˳����ȷ���ǣ�������| A�� | c��Na+����c��HA-����c��OH-����c��H2A����c��H+����c��A2-�� | B�� | c��Na+����c��HA-����c��H+����c��A2-����c��H2A����c��OH-�� | ||
| C�� | c��Na+����c��H+����c��HA-����c��A2-����c��OH-����c��H2A�� | D�� | c��Na+����c��OH-����c��HA-����c��H2A����c��H+����c��A2-�� |
���� ��V��NaOH��=20mLʱ��n��H2A��=n��NaOH��=0.2mol/L��0.02L=0.004mol������ǡ�÷�Ӧ����NaHA������ͼ֪����Һ��c��HA-����c��A2-����c��H2A����˵��H2A�Ƕ�Ԫ���ᣬc��A2-����c��H2A��˵��HA-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�����ԣ����Դ���c��H+����c��OH-����HA-�ĵ����ˮ��̶ȶ���С���ݴ˷������
��� �⣺��V��NaOH��=20mLʱ��n��H2A��=n��NaOH��=0.2mol/L��0.02L=0.004mol������ǡ�÷�Ӧ����NaHA������ͼ֪����Һ��c��HA-����c��A2-����c��H2A����˵��H2A�Ƕ�Ԫ���ᣬc��A2-����c��H2A��˵��HA-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�����ԣ����Դ���c��H+����c��OH-����HA-�ĵ����ˮ��̶ȶ���С�������Ӳ�ˮ�⣬����c��Na+����c��HA-����ˮ���������룬ֻ��HA-��������A2-������c��H+����c��A2-����ֻ��HA-ˮ������H2A����Һ�����ԣ�����������Ũ����С����������Ũ�ȴ�С˳����c��Na+����c��HA-����c��H+����c��A2-����c��H2A����c��OH-����
��ѡB��
���� �������������Һ�����ж�Ϊ���忼������Ũ�ȴ�С�Ƚϣ����ؿ���ѧ�������ж���������ȷ�����Һ�е����ʼ��������ǽⱾ��ؼ������ͼ�и�����Ũ�ȴ�С˳����������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ��Y����������ϼ�Ϊ+6 | B�� | �����Ӱ뾶�Ĵ�С˳��X��Y��Z | ||
| C�� | ����̬�⻯����ȶ��ԣ�X��Y | D�� | Ԫ��X��Y���γ����ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢܢ� | C�� | �ۢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
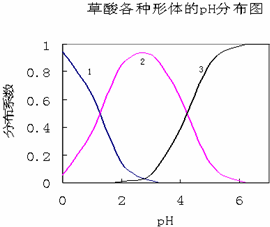 ���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | b-m-n | B�� | b+m-n | C�� | b-m+n | D�� | b+m+n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����м���Խ����Խ���������Խ�ȶ� | |
| B�� | P4��CH4�������������ͽṹ�ķ��ӣ��Ҽ��Ƕ�Ϊ109��28�@ | |
| C�� | �ڻ�ѧ��Ӧ�У�ijԪ���ɻ���̬��Ϊ����̬�����Ԫ��һ������ԭ | |
| D�� | ���Ӳ�ṹ��ͬ�ļ����ӣ���뾶��˵�����������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ��֬������������������ | B�� | ʯ���ѻ����ѽⶼ���Ƶ�ϩ�� | ||
| C�� | ��ȩ��֬�Ƿ���ȩ�����۲��� | D�� | ��ѿ�Ǻ����ǵ�ˮ�������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���ʱ��Դ������Ӧ���ص�X������ | |
| B�� | �ŵ�ʱ�����缫��ӦʽΪ��XH6+6e-�TX+6H+ | |
| C�� | ���ʱ�����缫��ӦʽΪ��6Ni��OH��2+6OH-�T6NiOOH+6H2O+6e- | |
| D�� | �õ�طŵ練Ӧ1 mol XH6ʱ�������ڵ��ˮ�������Ͽɵõ�16g���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ڿ�����ȼ�յĻ���ʻ�ɫ������Ϊ���ɵ�Na2O2Ϊ����ɫ���� | |
| B�� | ��˿��Cl2��ȼ���к���ɫ���̣�����Ϊ���ɵ�FeCl3Ϊ����ɫ���� | |
| C�� | ������������ϡHNO3����ַ�Ӧ����KSCN��Һ����Һ�ʺ�ɫ��˵��ϡHNO3��Fe����ΪFe3+ | |
| D�� | ȡ������ҺX�������м����������Ƶ���ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com