

| Fe2+ | Fe3+ | Al3+ | Mg2+ | |
| ��ʼ������pH | 7.5 | 2.8 | 4.2 | 9.6 |
| ������ȫʱ��pH | 9.0 | 4.0 | 5 | 11 |
���� ����������ܽ⣬�����Ȼ�����������������������������Ϊ�����ӣ�����pH��ʹ������ˮ�����������������������ˣ��õ������������壬���գ��õ���������
��1�����������֪����ʵ��Ҫ����������á����顱�ijɷ֣���Ҫͨ��ʵ�����̣�����Ԫ����ȫ�����������ݱ����֪��Ҫʹ��Ԫ����ȫ����������þ���������轫Fe2+ת��ΪFe3+��Ȼ�������
��2������11.2g�������е���Ԫ������ȫ��ת����M�У�������ԭ�ӵ��غ������㣬������Ԫ�ص�����������������
��������ù��˹���ֱ��ϴ�ӡ���ɡ����������㡰���顱�Ĵ��ȣ���ʱӦ����Fe��OH��3�ķֽ⣬���������ƫС��
��� �⣺����������ܽ⣬�����Ȼ�����������������������������Ϊ�����ӣ�����pH��ʹ������ˮ�����������������������ˣ��õ������������壬���գ��õ���������
��1�����ݱ����֪��Ҫʹ��Ԫ����ȫ�����������Ӻ�þ���Ӳ��������轫Fe2+ת��ΪFe3+������Ҫ����H2O2��
Ȼ�����4��pH��4.2���ɽ�Fe3+������Al3+��Mg2+������������pHѡ�õ��Լ�����������������Ϊ�������ɫ��δM������ƫ��Ҳ������MgCO3���壬��ΪMgCO3�������ʱ���������ù���M������ƫ��Ӧѡ�ð�ˮ��
�ʴ�Ϊ��C��D��
��2�����պ�ɫ��δMΪFe2O3������10.0g�������е���Ԫ������ȫ����11.2gFe2O3�У�����ԭ�ӵ�����Ϊ��m��Fe��=$\frac{11.2g}{160g/mol}$��2��56gmol=7.84g��
�ʸá����顱�Ĵ���=$\frac{7.84g}{10.0g}$��100%=78.4%��
��������ù��˹���ֱ��ϴ�ӡ���ɡ���������ɹ����л�����Fe��OH��3�ķֽ⣬����M���������ƫС���������FeԪ�ص�����ƫС���ʻᵼ�¼�����ƫС��
�ʴ�Ϊ��78.4%��Fe��OH��3�ں�ɹ����л��в��ַ����ֽⷴӦ��������С��
���� ���⿼�������ʺ����IJⶨ�Լ����ʵķ����ᴿ����Ŀ�Ѷ��еȣ�ע����յ���pH������������ӵķ����Լ�ѡ�õ��Լ����ܶ�ʵ��������ţ�


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v����v����v�� | B�� | v����v����v�� | C�� | v����v����v�� | D�� | v��=v��=v�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeCl3 | B�� | CuS | C�� | Na2O2 | D�� | FeS |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ڶ�ʮ�Ž����ƥ���ļ��˶�����2008��8��8��-24���ڱ���¡�ؾ��в���þ�ɹ�����ͼ�ǿ��ư���ƥ�������廷��һ���л������֮Ϊ����ƥ����������˵����ȷ���ǣ�������
�ڶ�ʮ�Ž����ƥ���ļ��˶�����2008��8��8��-24���ڱ���¡�ؾ��в���þ�ɹ�����ͼ�ǿ��ư���ƥ�������廷��һ���л������֮Ϊ����ƥ����������˵����ȷ���ǣ�������| A�� | ���л����һ�ȴ��ﹲ������ | |
| B�� | ���л�����ֻ���Ǽ��Լ��ķǼ��Է��� | |
| C�� | ���л������ڷ����廯����DZ���ͬϵ�� | |
| D�� | ���л�����ȫȼ������H2O�����ʵ���С��CO2�����ʵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
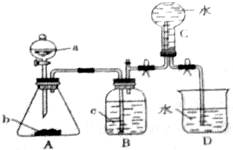
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��д��������Ӧ�Ļ�ѧ����ʽ��
��д��������Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����KMnO4��Һ������ᣨH2C2O4����Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�Ŀ������о�Ӱ�췴Ӧ���ʵ����أ�
����KMnO4��Һ������ᣨH2C2O4����Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�Ŀ������о�Ӱ�췴Ӧ���ʵ����أ�| ʵ�� ��� | �¶� ���棩 | ���� ������g�� | ����KMnO4��Һ | ʵ��Ŀ�� a��ʵ��1��2̽��___________ b��ʵ��1��3̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�� c��ʵ��1��4̽�������Ը÷�Ӧ���ʵ�Ӱ�� | |
| ��� ��mL�� | Ũ�� ��mol/L�� | ||||
| 1 | 25 | 0.5 | 4 | 0.1000 | |
| 2 | 50 | 0.5 | 4 | 0.1000 | |
| 3 | 25 | 0.5 | 4 | 0.0100 | |
| 4 | 25 | 0 | 4 | 0.1000 | |
| ʵ�� ��� | ��Һ��ɫ����ʱ�䣨min�� | ||
| ��1�� | ��2�� | ��3�� | |
| 1 | 14.0 | 13.0 | 11.0 |
| 3 | 6.5 | 6.7 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��A����A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ��ʧȥ���� | |
| B�� | ԭ�Ӽ������ӵĺ�����Ӳ������ڸ�Ԫ�����ڵ������� | |
| C�� | Ԫ�����ڱ��дӢ�B�嵽��B��10�����е�Ԫ�ض��ǽ���Ԫ�� | |
| D�� | �������±�ص��ʵ��۷е����ԭ������������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com