


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ˮԡ |
| ˮԡ |
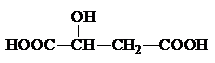 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ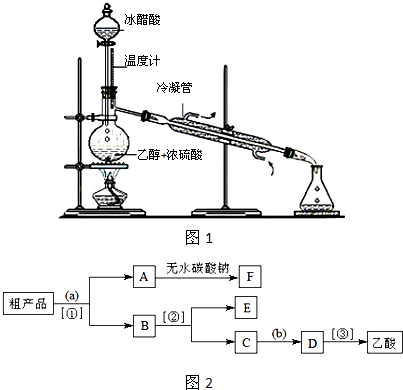
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
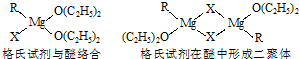
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��
��������²�ͬ����ش�
��1����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԡ�
��A�������еĻ�ѧ����_____________��
�ڽ�4.48 L����״���£�Xͨ��100mL 3 mol��L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳�� Ϊ_______________________________________��
Ϊ_______________________________________��
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100mL��Һ����������н��������ӵ�Ũ��Ϊ0.5 mol��L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ__________��
��2����AΪ��̬�ǽ������ʣ�A��Xͬ���ڣ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e �ṹ��
�ṹ��
�������й�B���ʵ�������ȷ����
a��B�ķ���ʽΪAX b��BΪ���ۻ�����
c��B���ӳ������� d��B�����ȶ��������X����κ����ʷ�����ѧ��Ӧ
��C��ˮ���ҷ�Ӧ���������ֳ����ᣬ��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݵ�����У�����߶��ڶ�ѧ�����л�ѧ�Ծ� ���ͣ������
��10�֣��ڳ����£���һ��Ũ�ȵ�CH3COOH��Һ�ζ�V mLͬŨ��NaOH��Һʱ�õ��ĵζ���������ͼ��
���ⶨij��Һ��ֻ����Na+��CHCOO����H+��OH�� �������ӣ���֪������Һ����һ�ֻ��������ʡ������ϱ�����Ũ�ȵ�CH3COOH��CH3COONa�Ļ��Һ�����ԡ���������и��⣺
��1���Է�����ͼ����ʾ�ζ����̵�b��d������ܵ�������ϣ�
b��_____________________��d��____________________��
��2���ֱ�ָ����ͼa��c���������ڵ���������Ũ�ȴ�С��ϵ��
a�㣺_________________________________________________________________________
c�㣺_________________________________________________________________________
��3��ˮ�ĵ���̶�����Һ�����ܽ�ĵ�����йأ��Է�����ͼa��b��c��d�㣬ˮ�ĵ���̶�������______��
��4���й�������Һ�����е�˵������ȷ����_________
| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com