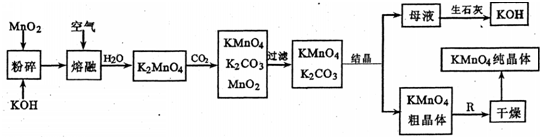
NaClO��Һ��һ�ֳ��õĻ�������Һ��ij��ѧ�о���ѧϰС������ϵõ��������ռ����¶�70
0C������Ҫ��������Ӧ��3Cl
2+6NaOH=?NaClO
3+5NaCl+3H
2O��������������Ʊ�NaClO��Һ��װ�ã���ͼ��ʾ��

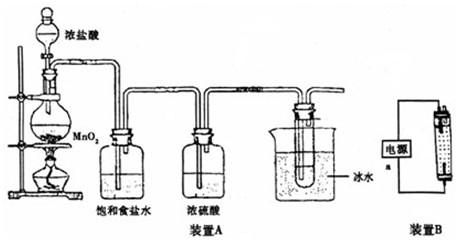
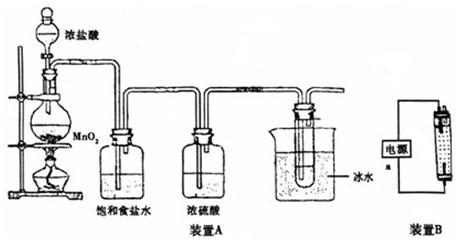
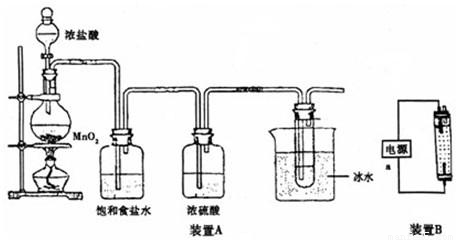
������װ��A�ش�
��1����ƿ�ڷ�Ӧ�Ļ�ѧ����ʽΪ
MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
�����Թ��ڷ�Ӧ�����ӷ���ʽΪ
Cl2+2OH-=Cl-+ClO-+H2O
Cl2+2OH-=Cl-+ClO-+H2O
��
��2������ʳ��ˮ��������
��ȥ��������HCl
��ȥ��������HCl
���ܷ�ʡȥʢŨ�����ϴ��ƿ�����ܻ��ܣ�
��
��
��װ��A������һ��������װ�ã�
β��������װ��
β��������װ��
��
������װ��B���缫��Ϊʯī���ش�
��3��װ��B��װ����Һ��
-NaCl
NaCl
��a�ӵ�Դ�ļ�
��
��
-������ܻ�ѧ�ķ���ʽΪ
��
��4�����һ��ʱ���ԭ������������ࣩȡ���Һ��������ɫʯ����ֽ�ϣ��۲쵽��������
�ȱ�������ɫ
�ȱ�������ɫ
��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�