
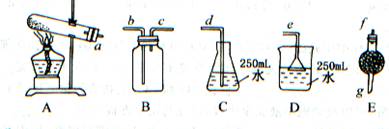
ȡwg�������ʣ��������Ȳ��ֽ⣩��NH4HCO3��ѡ����ͼ��ʾ��װ�ã���ȡһƿ����İ��������ఱ��ȫ����ˮ���ա��ش�
��1��ѡ�õ�װ���ǣ���A��B��C�����ش� ������ѡȡװ�õ���ȷ˳���ǣ�a��b��c������д���� �� �� �� �� �� ��
��2��Eװ������ʢҩƷ�������� ���������� ��
��3����ѡ��Bװ�ã�����ʱb�ڽ�������C�ڽ��� ����ԭ���� ��
��4�����ռ�������VmL����״��������ˮ��Ũ��Ϊa mol/L����̼����淋Ĵ���Ϊ
%����NH4HCO3ȫ���ֽ⣩��
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
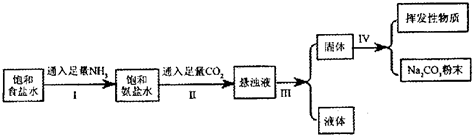
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
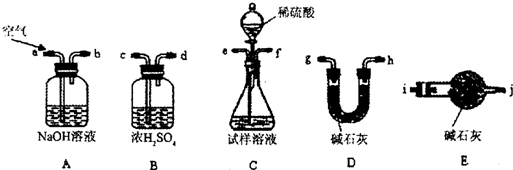
ȡwg�������ʣ��������Ȳ��ֽ⣩��NH4HCO3��ѡ����ͼ��ʾ��װ�ã���ȡһƿ����İ��������ఱ��ȫ����ˮ���ա��ش�
��1��ѡ�õ�װ���ǣ���A��B��C�����ش� ������ѡȡװ�õ���ȷ˳���ǣ�a��b��c������д���� �� �� �� �� �� ��
��2��Eװ������ʢҩƷ�������� ���������� ��
��3����ѡ��Bװ�ã�����ʱb�ڽ�������C�ڽ��� ����ԭ���� ��
��4�����ռ�������VmL����״��������ˮ��Ũ��Ϊa mol/L����̼����淋Ĵ���Ϊ
%����NH4HCO3ȫ���ֽ⣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ��пؽ���ѧ��һ�ڶ�ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
ȡwg�������ʣ��������Ȳ��ֽ⣩��NH4HCO3��ѡ����ͼ��ʾ��װ�ã���ȡһƿ����İ��������ఱ��ȫ����ˮ���ա��ش�
��1��ѡ�õ�װ���ǣ���A��B��C�����ش� ������ѡȡװ�õ���ȷ˳���ǣ�a��b��c������д���� �� �� �� �� �� ��
��2��Eװ������ʢҩƷ�������� ���������� ��
��3����ѡ��Bװ�ã�����ʱb�ڽ�������C�ڽ��� ����ԭ���� ��
��4�����ռ�������VmL����״��������ˮ��Ũ��Ϊa mol/L����̼����淋Ĵ���Ϊ
%����NH4HCO3ȫ���ֽ⣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ��и�һ�ڶ�ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
ȡwg�������ʣ��������Ȳ��ֽ⣩��NH4HCO3��ѡ����ͼ��ʾ��װ�ã���ȡһƿ����İ��������ఱ��ȫ����ˮ���ա��ش�
��1��ѡ�õ�װ���ǣ���A��B��C�����ش� ������ѡȡװ�õ���ȷ˳���ǣ�a��b��c������д���� �� �� �� �� �� ��
��2��Eװ������ʢҩƷ�������� ���������� ��
��3����ѡ��Bװ�ã�����ʱb�ڽ�������C�ڽ��� ����ԭ���� ��
��4�����ռ�������VmL����״��������ˮ��Ũ��Ϊa mol/L����̼����淋Ĵ���Ϊ
%����NH4HCO3ȫ���ֽ⣩��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com