
��11�֣��ڻ���ƽ�����װ��ǿ��ԭ���£�N2H4����ǿ��������H2O2���������ǻ��ʱ��������������N2��ˮ���������ų������ȡ���֪0.4molҺ̬ �º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65kJ��������
�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65kJ��������
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________��
��2����֪H2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
��3��������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���________________________��
��4����֪N2(g)+2O2(g)="===2" NO2(g)����H="+67.7" kJ��mo l-1�� N2H4(g)+O2(g)="==" N2(g)+2H2O (g)����H="-534" kJ��mol-1�����ݸ�˹����д������NO2��ȫ��Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ________________________________________��
l-1�� N2H4(g)+O2(g)="==" N2(g)+2H2O (g)����H="-534" kJ��mol-1�����ݸ�˹����д������NO2��ȫ��Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ________________________________________��
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
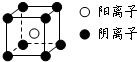 ������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺
������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺



| ||
| 2 |
| 3 |
| ||
| ||
| 2 |
| 3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
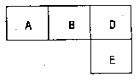 ��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ����������壬NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ�A��B��D��E�ֱ��ڱ������ڱ���һ���֣�ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37���Իش�
��M��A��B��D��N��E���ֶ�����Ԫ�أ�ԭ��������������MԪ�صĵ�������Ȼ����������壬NԪ�ص�ԭ�Ӱ뾶����������ԭ�Ӱ뾶���ģ�A��B��D��E�ֱ��ڱ������ڱ���һ���֣�ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37���Իش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com