
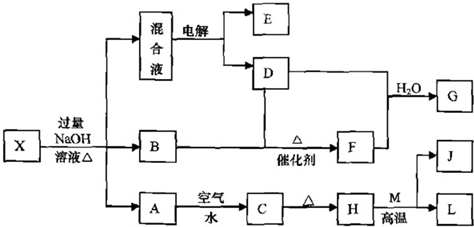
��4�֣���֪2NH3(g)  N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
| ���� | NH3 | N2 | H2 |
| ���ʵ�����mol�� | 0. 1 | 0.2 | 0.6 |
 ������ʹ����ƽ���ƶ�
������ʹ����ƽ���ƶ�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
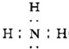

| 2b |
| a |
| 2b |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������2010��2011ѧ���һ��ѧ�����п��Ի�ѧ���� ���ͣ�022
��֪2NH3(g)![]() N2(g)��3H2(g)����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g)��3H2(g)����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
![]()
��ʱ����ƽ����Ӧ����v(N2)��________
�ﵽƽ��״̬������˵����ȷ���ǣ�________
a��ͨ���ı䷴Ӧ������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣���֪2NH3(g) N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
| ���� | NH3 | N2 | H2 |
| ���ʵ�����mol�� | 0. 1 | 0.2 | 0.6 |
��ʱ����ƽ����Ӧ����v(N2) ��
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2�� H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��4�֣���֪2NH3(g)  N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
|
���� |
NH3 |
N2 |
H2 |
|
���ʵ�����mol�� |
0. 1 |
0.2 |
0.6 |
��ʱ����ƽ����Ӧ����v(N2) ��
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2�� H2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com