
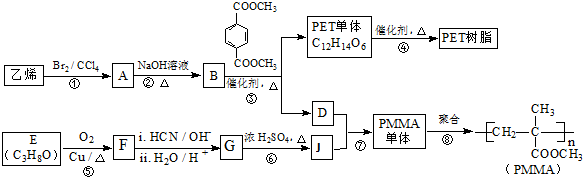
 ŅŃÖŖ£ŗ
ŅŃÖŖ£ŗ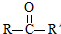 $”ś_{ii£®H_{2}O/H+}^{i£®HCN/OH-}$
$”ś_{ii£®H_{2}O/H+}^{i£®HCN/OH-}$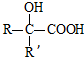 £ØR”¢R”Æ“ś±ķĢž»ł£©
£ØR”¢R”Æ“ś±ķĢž»ł£© £®
£® £®
£® £®
£®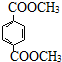 Óė×ćĮæNaOHČÜŅŗ·“Ó¦Ź±£¬×ī¶ąĻūŗÄ4mol NaOH
Óė×ćĮæNaOHČÜŅŗ·“Ó¦Ź±£¬×ī¶ąĻūŗÄ4mol NaOH·ÖĪö øł¾ŻĢāÖŠø÷ĪļÖŹ×Ŗ»Æ¹ŲĻµ£¬ÓÉPMMAµÄ½į¹¹æÉÖŖ£¬PMMAµ„ĢåĪŖCH2=C£ØCH3£©COOCH3£¬EŃõ»ÆµĆF£¬FµÄŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾Ö»ÓŠŅ»×é·å£¬F·¢ÉśŠÅĻ¢¢ņÖŠµÄ·“Ó¦µĆG£¬GŌŚÅØĮņĖį×÷ÓĆĻĀ·¢ÉśĻūČ„·“Ó¦µĆJ£¬½įŗĻPMMAµ„ĢåµÄ½į¹¹ŗĶEµÄ·Ö×ÓŹ½æÉÖŖ£¬EĪŖCH3CHOHCH3£¬FĪŖCH3COCH3£¬GĪŖ£ØCH3£©2COHCOOH£¬JĪŖCH2=C£ØCH3£©COOH£¬ĖłŅŌDĪŖHOCH3£¬ŅŅĻ©Óėäå·¢Éś¼Ó³É·“Ӧɜ³ÉAĪŖBrCH2CH2Br£¬AŌŚ¼īŠŌĢõ¼žĻĀĖ®½āµĆBĪŖHOCH2CH2OH£¬BÓė¶Ō±½¶ž¼×Ėį¼×õ„·¢ÉśČ”“ś·“Ӧɜ³ÉPETµ„ĢåĪŖ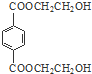 £¬PETµ„Ģå·¢ÉśŠÅĻ¢¢ńµÄ·“Ó¦µĆPET¾Ūõ„£¬¾Ż“Ė“šĢā£®
£¬PETµ„Ģå·¢ÉśŠÅĻ¢¢ńµÄ·“Ó¦µĆPET¾Ūõ„£¬¾Ż“Ė“šĢā£®
½ā“š ½ā£ŗøł¾ŻĢāÖŠø÷ĪļÖŹ×Ŗ»Æ¹ŲĻµ£¬ÓÉPMMAµÄ½į¹¹æÉÖŖ£¬PMMAµ„ĢåĪŖCH2=C£ØCH3£©COOCH3£¬EŃõ»ÆµĆF£¬FµÄŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾Ö»ÓŠŅ»×é·å£¬F·¢ÉśŠÅĻ¢¢ņÖŠµÄ·“Ó¦µĆG£¬GŌŚÅØĮņĖį×÷ÓĆĻĀ·¢ÉśĻūČ„·“Ó¦µĆJ£¬½įŗĻPMMAµ„ĢåµÄ½į¹¹ŗĶEµÄ·Ö×ÓŹ½æÉÖŖ£¬EĪŖCH3CHOHCH3£¬FĪŖCH3COCH3£¬GĪŖ£ØCH3£©2COHCOOH£¬JĪŖCH2=C£ØCH3£©COOH£¬ĖłŅŌDĪŖHOCH3£¬ŅŅĻ©Óėäå·¢Éś¼Ó³É·“Ӧɜ³ÉAĪŖBrCH2CH2Br£¬AŌŚ¼īŠŌĢõ¼žĻĀĖ®½āµĆBĪŖHOCH2CH2OH£¬BÓė¶Ō±½¶ž¼×Ėį¼×õ„·¢ÉśČ”“ś·“Ӧɜ³ÉPETµ„ĢåĪŖ £¬PETµ„Ģå·¢ÉśŠÅĻ¢¢ńµÄ·“Ó¦µĆPET¾Ūõ„£¬
£¬PETµ„Ģå·¢ÉśŠÅĻ¢¢ńµÄ·“Ó¦µĆPET¾Ūõ„£¬
£Ø1£©øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖ£¬·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶŹĒ¼Ó³É·“Ó¦£¬
¹Ź“š°øĪŖ£ŗ¼Ó³É·“Ó¦£»
£Ø2£©¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ  £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø3£©·“Ó¦¢ŻµÄ»Æѧ·½³ĢŹ½ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø4£©øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖ£¬GµÄ½į¹¹¼ņŹ½ĪŖ  £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø5£©a.1mol  Óė×ćĮæNaOHČÜŅŗ·“Ó¦Ź±£¬×ī¶ąĻūŗÄ2mol NaOH£¬¹Źa“ķĪó£»
Óė×ćĮæNaOHČÜŅŗ·“Ó¦Ź±£¬×ī¶ąĻūŗÄ2mol NaOH£¬¹Źa“ķĪó£»
b£®DĪŖHOCH3£¬BĪŖHOCH2CH2OH£¬ĖüĆĒµÄōĒ»łµÄŹżÄæ²»Ķ¬£¬ĖłŅŌBŗĶD²»ŹĒ»„ĪŖĶ¬ĻµĪļ£¬¹Źb“ķĪó£»
c£®¢ßĪŖCH2=C£ØCH3£©COOHÓėHOCH3·¢Éśõ„»Æ·“Ó¦£¬¹ŹcÕżČ·£»
d£®DÖŠÓŠōĒ»ł£¬ÄÜŠĪ³ÉĒā¼ü£¬ĖłŅŌDµÄ·Šµć±ČĶ¬Ģ¼Ō×ÓŹżµÄĶéĢžøߣ¬¹ŹdÕżČ·£»
¹ŹŃ”cd£»
£Ø6£©JµÄijÖÖĶ¬·ÖŅģ¹¹ĢåÓėJ¾ßÓŠĻąĶ¬¹ŁÄÜĶÅ£¬ĘäÖŠĪŽ¼×»łµÄŅ»ÖÖ½į¹¹¼ņŹ½ŹĒCH2=CHCH2COOH£¬
¹Ź“š°øĪŖ£ŗCH2=CHCH2COOH£»
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻÓėŗĻ³É£¬×¢Ņāøł¾Ż³ä·ÖĄūÓĆĢāÖŠŠÅĻ¢ŗĶÓŠ»śĪļµÄ½į¹¹½ųŠŠĶʶĻ£¬Ć÷Č·ÓŠ»śĪļµÄ¹ŁÄÜĶż°ĘäŠŌÖŹŹĒ½ā±¾Ģā¹Ų¼ü£¬ÄѶČÖŠµČ£®


 ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø
ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø »ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
»ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | »ģŗĻĘųĢåµÄĆܶČŗć¶Ø²»±ä | B£® | »ģŗĻĘųĢåµÄŃÕÉ«²»ŌŁøıä | ||
| C£® | H2”¢I2”¢HIµÄÅضČĻąµČ | D£® | ĘųĢå×ÜĪļÖŹµÄĮæ²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
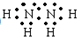 £¬¹żŃõ»ÆĒāµÄ½į¹¹Ź½£ŗ
£¬¹żŃõ»ÆĒāµÄ½į¹¹Ź½£ŗ £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4Cl£Øs£©=NH3£Øg£©+HCl£Øg£©ŹŅĪĀĻĀ²»ÄÜ×Ō·¢½ųŠŠ£¬ĖµĆ÷øĆ·“Ó¦µÄ”÷H£¼0 | |
| B£® | ¶ĘĪżĢśÖĘĘ·¶Ę²ćĘĘĖšŗó£¬ĢśÖĘĘ·±ČŹÜĖšĒ°øüČŻŅ×ÉśŠā£¬¶ų¶ĘŠæĢśÖĘĘ·ŌņĻą·“ | |
| C£® | ½«“æĖ®¼ÓČČÖĮ½ĻøßĪĀ¶Č£¬K±ä“ó”¢pH±äŠ””¢³ŹĖįŠŌ | |
| D£® | øų0.1mol•L-1CH3COOHČÜŅŗÖŠ¼ÓČČ£¬Ōņc£ØH+£©Óėc£ØCH3COOH£©µÄ±ČÖµŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ©”¢±ūĻ© | B£® | ¼×“¼”¢ŅŅ¶ž“¼ | C£® | ŅŅČ²”¢±½ | D£® | ±ūĻ©”¢»·±ūĶé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOH | B£® | AlCl3 | C£® | K2S | D£® | Cl2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā


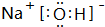 £» ÓƵē×ÓŹ½±ķŹ¾GÓėQŠĪ³É»ÆŗĻĪļµÄ¹ż³Ģ
£» ÓƵē×ÓŹ½±ķŹ¾GÓėQŠĪ³É»ÆŗĻĪļµÄ¹ż³Ģ £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆNaClÅä³ÉŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗ | B£® | HClČÜÓŚĖ® | ||
| C£® | Ė®±ä³ÉĖ®ÕōĘų | D£® | £ØNH4£©2CO3¼ÓĒæČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2S | B£® | CaCl2 | C£® | Na2O2 | D£® | H2O2 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com