


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
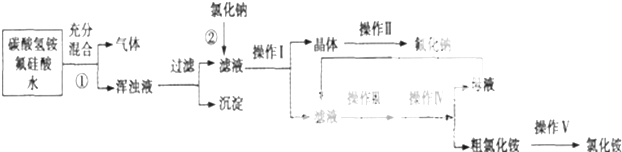
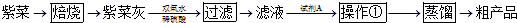
| �Ҵ� | ���Ȼ�̼ | �ѻ����� | �⣨���壩 | |
| �ܶ�/g��cm-3 | 0.7893 | 1.595 | 0.71��0.76 | 4.94 |
| �е�/�� | 78.5 | 76.8 | 25��232 | 184.35 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
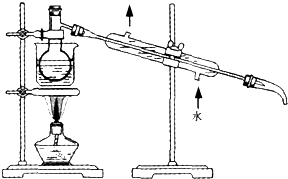
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�
������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�
| ||
| ���� |
| �� |
| ���� |
| �� |
| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
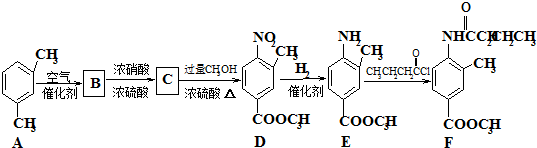
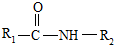 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ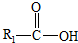 ��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ���� ����/g | ����״̬ | C��H2SO4�� /mol?L-1 | V��H2SO4�� /mL | ��Һ�¶�/�� | ������ʧ��ʱ��/s | |
| ��Ӧǰ | ��Ӧ�� | ||||||
| 1 | 0.10 | ˿ | 0.5 | 50 | 20 | 34 | 500 |
| 2 | 0.10 | ��ĩ | 0.5 | 50 | 20 | 35 | 50 |
| 3 | 0.10 | ˿ | 0.7 | 50 | 20 | 36 | 250 |
| 4 | 0.10 | ˿ | 0.8 | 50 | 20 | 35 | 200 |
| 5 | 0.10 | ��ĩ | 0.8 | 50 | 20 | 36 | 25 |
| 6 | 0.10 | ˿ | 1.0 | 50 | 20 | 35 | 125 |
| 7 | 0.10 | ˿ | 1.0 | 50 | 35 | 50 | 50 |
| 8 | 0.10 | ˿ | 1.1 | 50 | 20 | 34 | 100 |
| 9 | 0.10 | ˿ | 1.1 | 50 | 20 | 44 | 40 |
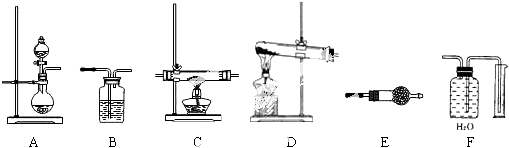
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
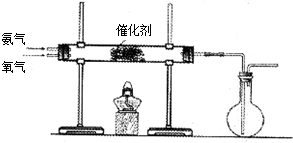
| O2 |
| O2 |
| H2O |
| A���٢� | B���ۢ� |
| C���٢ڢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com