
| ||
| 1min |
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaHS��ˮ�еĵ��뷽��ʽΪ��NaHS�TNa++HS-��HS-�TH++S2- |
| B��ͬ���ʵ���Ũ�ȵİ�ˮ�����ᷴӦ������ʱ���������V��NH3?H2O����V��HCl�� |
| C��Na2SO3��Һ�У�c��H+��+c��HSO3-��+2c��H2SO3��=c��OH-�� |
| D��ͬŨ�ȵ�������Һ�У�c��CH3COO-���Ĵ�С��CH3COONa��CH3COONH4��CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�� ������25�棩 |
1.8��l0-5 | 4.9��l0-10 | K1=4.3��l0-7 K2=5.6��l0-11 |
| A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪpH��NaCN����pH��Na2CO3����pH��CH3COONa�� |
| B��amol?L-1HCN��Һ��amol?L-1NaOH��Һ�������Ϻ�������Һ�Լ��ԣ�pH��7������c ��OH-����c��H+����c��Na+����c��CN-�� |
| C������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С |
| D��NaHCO3��Na2CO3�����Һ�У�һ����c��Na+��+c��H+��=c��OH-��+c��HCO3-��+c��CO32-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
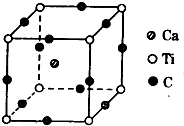 ������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ�
������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | |
| ����� | ������ | ��ɢϵ | |
| �����ڸ��������� | Na2CO3 |
| ����� | �ܵ�������� | ����� | ǿ����� |
| ���ڸ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 W��X��Y��Z��RΪǰ������Ԫ����ԭ��������������W��Y�γɳ����³�Һ̬�Ļ�����G��X����spm��m=1��2��3���ӻ���ʽ��X�γ�һϵ��X�Ļ����X�ĵ����ڸ�����������G��Ӧ����W���ʺ���̬�����W�ĵ�����Z�ĵ����ڵ�ȼ�����ʱ���ܷ�Ӧ����1��1��ˮ��ҺΪ�������Rԭ�ӵļ۵��Ӳ��δ�ɶԵ�����Ϊ4���ش��������⣺
W��X��Y��Z��RΪǰ������Ԫ����ԭ��������������W��Y�γɳ����³�Һ̬�Ļ�����G��X����spm��m=1��2��3���ӻ���ʽ��X�γ�һϵ��X�Ļ����X�ĵ����ڸ�����������G��Ӧ����W���ʺ���̬�����W�ĵ�����Z�ĵ����ڵ�ȼ�����ʱ���ܷ�Ӧ����1��1��ˮ��ҺΪ�������Rԭ�ӵļ۵��Ӳ��δ�ɶԵ�����Ϊ4���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
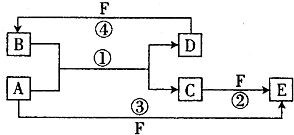
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ��������С��X��Y |
| B��Xm+��Yn-���Ӱ뾶��С��Yn-��Xm+ |
| C�������ڱ���X��Y�ڲ�ͬ���� |
| D�������ڱ���X��Y��������֮��Ϊ��8-��m+n�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com