
£Ø14·Ö£©ÖŠŗĶČČµÄ²ā¶ØŹµŃéµÄ¹Ų¼üŹĒŅŖ±Č½Ļ×¼Č·µŲÅäÖĘŅ»¶ØµÄĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ŌŚŹµŃé¹ż³ĢÖŠŅŖ¾”Įæ±ÜĆāČČĮæµÄÉ¢Ź§£¬ŅŖĒó±Č½Ļ×¼Č·µŲ²āĮæ³ö·“Ó¦Ē°ŗóČÜŅŗĪĀ¶ČµÄ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
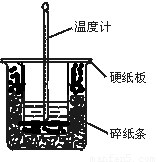
£Ø1£©“ÓŹµŃé×°ÖĆÉĻæ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄŅ»ÖÖ²£Į§ÓĆĘ·ŹĒ ”£
£Ø2£©ŌŚ“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄ£Ø»ņÖ½Ģõ£©Ęä×÷ÓĆŹĒ____________________£»“óÉÕ±ÉĻČē²»øĒÓ²Ö½°å£¬ĒóµĆµÄÖŠŗĶČČŹżÖµ (Ģī”°Ę«“ó”¢Ę«Š””¢ĪŽÓ°Ļģ”±)
£Ø3£©×öŅ»“ĪĶźÕūµÄÖŠŗĶČČŹµŃ飬ĪĀ¶Č¼ĘŠčŅŖŹ¹ÓĆ______ “Ī£»
£Ø4£©øĆŹµŃé³£ÓĆ0£®50 mol”¤L£1 HClŗĶ0£®55 mol”¤L£1µÄNaOHČÜŅŗø÷50 mL”£ČōÉĻŹöHCl”¢NaOHČÜŅŗµÄĆܶȶ¼½üĖĘĪŖ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉµÄČÜŅŗµÄ±ČČČČŻC = 4.18 J/£Øg”¤”ę£©£¬·“Ó¦ŗóĪĀ¶ČÉżøßĮĖ”÷t, ŌņÉś³É1molĖ®Ź±µÄ·“Ó¦ČȦ¤H=___________ kJ/mol£ØĢī±ķ“ļŹ½£©”£
£Ø5£©Čē¹ūÓĆ50mL0.50mol/L“×ĖįÓė50mL0.55mol/LNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ (Ģī”°ĻąµČ”¢²»ĻąµČ”±)£¬¼ņŹöĄķÓÉ ”£
£Ø1£© »·ŠĪ²£Į§½Į°č°ō £Ø2£©±£ĪĀ”¢øōČČ£»Ę«Š” £Ø3£©3 £»
£Ø4£©£0.418”÷t/0.025 £Ø5£©²»ĻąµČ£»ŅņĪŖ“×ĖįµēĄėŠčŅŖĪüŹÕČČĮ攣
”¾½āĪö”æ£Ø1£©ŌŚ²ā¶ØÖŠŗĶČȵďµŃéÖŠĪŖĮĖŹ¹ČÜŅŗ»ģŗĻ¾łÓŠ£¬ŌŚŹµŃé¹ż³ĢÖŠŠčŅŖ½Į°č£¬øł¾Ż×°ÖĆĶ¼æÉÖŖ»¹Č±ÉŁ»·ŠĪ²£Į§½Į°čĘ÷”£
£Ø2£©ĪŖĮĖ¼õÉŁČČĮæµÄĖšŹ§ŠčŅŖŌŚ“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄŅŌĘšµ½±£ĪĀŗĶøōČȵÄ×÷ÓĆ”£Čē¹ūƻӊøĒÓ²ÖŹ°å£¬»įŌģ³ÉČĖĄąµÄĖšŹ§£¬µ¼ÖĀ²ā¶Ø½į¹ūĘ«Š””£
£Ø3£©ŃĪĖįŗĶĒāŃõ»ÆÄĘČÜŅŗµÄĪĀ¶Č¶¼ŠčŅŖ²āĮ棬·“Ó¦ŗ󻹊čŅŖ²āĮæ»ģŗĻŅŗµÄĪĀ¶Č£¬ĖłŅŌÖĮÉŁŹ¹ÓĆ3“Ī”£
£Ø4£©50mL0.50mol/LŃĪĖįÓė50mL0.55mol/LNaOHČÜŅŗµÄÖŹĮæÖ®ŗĶŹĒ100g£¬ĖłŅŌ·“Ó¦¹ż³ĢÖŠ·Å³öµÄČČĮæŹĒ100g”Į4.18J/(g•”ę£©”Į”÷t”ę£½0.418”÷tkJ”£ŅņĪŖ50mL0.50mol/LŃĪĖįÓė50mL0.55mol/L
NaOHČÜŅŗ·“Ӧɜ³ÉĖ®ŹĒ0.025mol£¬ĖłŅŌ”÷H£½£0.418”÷t/0.025kJ/mol”£
£Ø5£©ŅņĪŖ“×ĖįŹĒČõĖįĆ»ŌŚČÜŅŗÖŠ“ęŌŚµēĄėĘ½ŗā£¬¶ųµēĄėŹĒĪüČČµÄ£¬ĖłŅŌ·Å³öµÄČČĮæŅŖÉŁ”£


 ½šÅĘ½ĢøØÅąÓÅÓÅŃ”¾ķĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
½šÅĘ½ĢøØÅąÓÅÓÅŃ”¾ķĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø14·Ö£©ÖŠŗĶČČµÄ²ā¶ØŹµŃéµÄ¹Ų¼üŹĒŅŖ±Č½Ļ×¼Č·µŲÅäÖĘŅ»¶ØµÄĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ŌŚŹµŃé¹ż³ĢÖŠŅŖ¾”Įæ±ÜĆāČČĮæµÄÉ¢Ź§£¬ŅŖĒó±Č½Ļ×¼Č·µŲ²āĮæ³ö·“Ó¦Ē°ŗóČÜŅŗĪĀ¶ČµÄ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©“ÓŹµŃé×°ÖĆÉĻæ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄŅ»ÖÖ²£Į§ÓĆĘ·ŹĒ ”£
£Ø2£©ŌŚ“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄ£Ø»ņÖ½Ģõ£©Ęä×÷ÓĆŹĒ____________________£»“óÉÕ±ÉĻČē²»øĒÓ²Ö½°å£¬ĒóµĆµÄÖŠŗĶČČŹżÖµ (Ģī”°Ę«“ó”¢Ę«Š””¢ĪŽÓ°Ļģ”±)
£Ø3£©×öŅ»“ĪĶźÕūµÄÖŠŗĶČČŹµŃ飬ĪĀ¶Č¼ĘŠčŅŖŹ¹ÓĆ______ “Ī£»
£Ø4£©øĆŹµŃé³£ÓĆ0£®50 mol”¤L£1 HClŗĶ0£®55 mol”¤L£1µÄNaOHČÜŅŗø÷50 mL”£ČōÉĻŹöHCl”¢NaOHČÜŅŗµÄĆܶȶ¼½üĖĘĪŖ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉµÄČÜŅŗµÄ±ČČČČŻC = 4.18 J/£Øg”¤”ę£©£¬·“Ó¦ŗóĪĀ¶ČÉżøßĮĖ”÷t, ŌņÉś³É1molĖ®Ź±µÄ·“Ó¦ČȦ¤H=___________kJ/mol£ØĢī±ķ“ļŹ½£©”£
£Ø5£©Čē¹ūÓĆ50mL0.50mol/L“×ĖįÓė50mL0.55mol/LNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ (Ģī”°ĻąµČ”¢²»ĻąµČ”±)£¬¼ņŹöĄķÓÉ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ°²»ÕŹ”ĶĮźŅ»ÖŠø߶ž10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ÖŠŗĶČČµÄ²ā¶ØŹµŃéµÄ¹Ų¼üŹĒŅŖ±Č½Ļ×¼Č·µŲÅäÖĘŅ»¶ØµÄĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ĮæČČĘ÷ŅŖ¾”Įæ×öµ½¾ųČČ£»ŌŚĮæČČµÄ¹ż³ĢÖŠŅŖ¾”Įæ±ÜĆāČČĮæµÄÉ¢Ź§£¬ŅŖĒó±Č½Ļ×¼Č·µŲ²āĮæ³ö·“Ó¦Ē°ŗóČÜŅŗĪĀ¶ČµÄ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖŠŃ§»ÆѧŹµŃéÖŠµÄÖŠŗĶČČµÄ²ā¶ØĖłŠčµÄ²£Į§ŅĒĘ÷ÓŠ£ŗ_________ ___________£¬ŌŚ“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄ£Ø»ņÖ½Ģõ£©Ęä×÷ÓĆŹĒ_____ _______________”£
£Ø2£©øĆŹµŃéæÉÓĆ0£®60 mol”¤L£1 HClŗĶ0£®65 mol”¤L£1µÄNaOHČÜŅŗø÷50 mL”£NaOHµÄÅØ¶Č“óÓŚHClµÄÅضČ×÷ÓĆŹĒ___ __”£µ±ŹŅĪĀµĶÓŚ10”ꏱ½ųŠŠ£¬¶ŌŹµŃé½į¹ū»įŌģ³É½Ļ“óµÄĪó²īĘäŌŅņŹĒ_________”£
£Ø3£©ČōÉĻŹöHCl”¢NaOHČÜŅŗµÄĆܶȶ¼½üĖĘĪŖ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉµÄČÜŅŗµÄ±ČČČČŻC=4£®18 J/£Øg”¤”ę£©£¬ŌņøĆÖŠŗĶ·“Ó¦·Å³öČČĮæĪŖ_____________kJ£ØĢī±ķ“ļŹ½£©£¬¦¤H="___________" kJ/mol£ØĢī±ķ“ļŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ°²»ÕŹ”ø߶ž10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ÖŠŗĶČČµÄ²ā¶ØŹµŃéµÄ¹Ų¼üŹĒŅŖ±Č½Ļ×¼Č·µŲÅäÖĘŅ»¶ØµÄĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ĮæČČĘ÷ŅŖ¾”Įæ×öµ½¾ųČČ£»ŌŚĮæČČµÄ¹ż³ĢÖŠŅŖ¾”Įæ±ÜĆāČČĮæµÄÉ¢Ź§£¬ŅŖĒó±Č½Ļ×¼Č·µŲ²āĮæ³ö·“Ó¦Ē°ŗóČÜŅŗĪĀ¶ČµÄ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖŠŃ§»ÆѧŹµŃéÖŠµÄÖŠŗĶČČµÄ²ā¶ØĖłŠčµÄ²£Į§ŅĒĘ÷ÓŠ£ŗ_________ ___________£¬ŌŚ“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄ£Ø»ņÖ½Ģõ£©Ęä×÷ÓĆŹĒ_____ _______________”£
£Ø2£©øĆŹµŃéæÉÓĆ0£®60 mol”¤L£1 HClŗĶ0£®65 mol”¤L£1µÄNaOHČÜŅŗø÷50 mL”£NaOHµÄÅØ¶Č“óÓŚHClµÄÅضČ×÷ÓĆŹĒ___ __”£µ±ŹŅĪĀµĶÓŚ10”ꏱ½ųŠŠ£¬¶ŌŹµŃé½į¹ū»įŌģ³É½Ļ“óµÄĪó²īĘäŌŅņŹĒ_________”£
£Ø3£©ČōÉĻŹöHCl”¢NaOHČÜŅŗµÄĆܶȶ¼½üĖĘĪŖ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉµÄČÜŅŗµÄ±ČČČČŻC=4£®18 J/£Øg”¤”ę£©£¬ŌņøĆÖŠŗĶ·“Ó¦·Å³öČČĮæĪŖ_____________kJ£ØĢī±ķ“ļŹ½£©£¬¦¤H=___________ kJ/mol£ØĢī±ķ“ļŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com