
½«H2SŗĶæÕĘųµÄ»ģŗĻĘųĢåĶØČėFeCl3”¢FeCl2”¢CuCl2µÄ»ģŗĻČÜŅŗÖŠ·“Ó¦»ŲŹÕS£¬ĘäĪļÖŹ×Ŗ»ÆČēĶ¼ĖłŹ¾”£
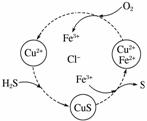
(1)ŌŚĶ¼Ź¾µÄ×Ŗ»ÆÖŠ£¬»ÆŗĻ¼Ū²»±äµÄŌŖĖŲŹĒ________”£
(2)·“Ó¦ÖŠµ±ÓŠ1 mol H2S×Ŗ»ÆĪŖĮņµ„ÖŹŹ±£¬±£³ÖČÜŅŗÖŠFe3£«µÄĪļÖŹµÄĮæ²»±ä£¬ŠčĻūŗÄO2µÄĪļÖŹµÄĮæĪŖ______”£
(3)ŌŚĪĀ¶ČŅ»¶ØŗĶ²»²¹¼ÓČÜŅŗµÄĢõ¼žĻĀ£¬»ŗĀżĶØČė»ģŗĻĘųĢ壬²¢³ä·Ö½Į°č”£ÓūŹ¹Éś³ÉµÄĮņµ„ÖŹÖŠ²»ŗ¬CuS£¬æɲÉČ”µÄ“ėŹ©ÓŠ____________________________________”£
“š°ø””(1)Cu”¢H”¢Cl(»ņĶ”¢Ēā”¢ĀČ)””(2)0.5 mol””(3)Ģįøß»ģŗĻĘųĢåÖŠæÕĘųµÄ±ČĄż
½āĪö””(1)øł¾ŻĶ¼Ź¾æÉÖŖ£¬Õūøö¹ż³ĢÖŠ£2¼ŪµÄSÓė0¼ŪµÄSÖ®¼äĻą»„×Ŗ»Æ£¬»¹“ęŌŚFe2£«ÓėFe3£«Ö®¼äµÄĻą»„×Ŗ»Æ”£(2)øł¾Żµē×ÓŹŲŗćŗĶ»Æѧ·“Ó¦·½³ĢŹ½ÖŖ£¬1 mol H2S×Ŗ»ÆĪŖµ„ÖŹS×ŖŅĘ2 molµē×Ó£¬¶ų1 mol O2×ŖŅĘ4 molµē×Ó£¬Ņņ“ĖŠčĻūŗÄO2 0.5 mol”£(3)øł¾ŻĶ¼Ź¾£¬æÉŌö“óæÕĘųµÄĮ棬Ź¹S2£ĶźČ«×Ŗ»ÆĪŖµ„ÖŹS£¬Ź¹Ęäƻӊ»ś»įŗĶCu2£«½įŗĻÉś³ÉCuS”£


 ·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³ÉµÄijӊ»ś»ÆŗĻĪļ8.8 g£¬ĶźČ«Č¼ÉÕŗóÉś³ÉCO2ŗĶH2OµÄÖŹĮæ·Ö±šŹĒ22.0 gŗĶ10.8 g£¬ŌņøĆ»ÆŗĻĪļµÄ·Ö×ÓŹ½ĪŖ(””””)
A£®C5H6O B£®C5H12O
C£®C5H12O2 D£®C5H10O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬ŌŚĻĀĮŠČÜŅŗÖŠ·¢ÉśČēĻĀ·“Ó¦£ŗ
¢Ł16H£«£«10Z££«2XO ===2X2£«£«5Z2£«8H2O
===2X2£«£«5Z2£«8H2O
¢Ś2A2£«£«B2===2A3£«£«2B£
¢Ū2B££«Z2===B2£«2Z£
ÓÉ“ĖÅŠ¶ĻĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®·“Ó¦Z2£«2A2£«===2A3£«£«2Z£²»ÄܽųŠŠ
B£®ZŌŖĖŲŌŚ¢Ł¢Ū·“Ó¦ÖŠ¾ł±»Ńõ»Æ
C£®Ńõ»ÆŠŌÓÉČõµ½ĒæµÄĖ³ŠņŹĒXO ”¢Z2”¢B2”¢A3£«
”¢Z2”¢B2”¢A3£«
D£®»¹ŌŠŌÓÉĒæµ½ČõµÄĖ³ŠņŹĒA2£«”¢B£”¢Z£”¢X2£«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ·“Ó¦3BrF3£«5H2O===9HF£«Br2£«HBrO3£«O2”üÖŠ£¬ČōÓŠ5 mol H2O²Ī¼Ó·“Ó¦£¬±»Ė®»¹ŌµÄäåŌŖĖŲĪŖ(””””)
A£®1 mol B.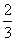 mol C.
mol C.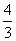 mol D£®2 mol
mol D£®2 mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗ¬ÓŠÅųĖŖ(As2O3)µÄŹŌŃłŗĶŠæ”¢ŃĪĖį»ģŗĻ·“Ó¦£¬Éś³ÉµÄÉé»ÆĒā(AsH3)ŌŚČČ²£Į§¹ÜÖŠĶźČ«·Ö½ā³Éµ„ÖŹÉéŗĶĒāĘų”£ČōÉéµÄÖŹĮæĪŖ1.50 mg£¬Ōņ(””””)
A£®±»Ńõ»ÆµÄÅųĖŖĪŖ1.98 mg
B£®·Ö½ā²śÉśµÄĒāĘųĪŖ0.672 mL
C£®ŗĶÅųĖŖ·“Ó¦µÄŠæĪŖ3.90 mg
D£®×ŖŅʵĵē×Ó×ÜŹżĪŖ6”Į10£5NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÅäĘ½·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
 Mn2£«£«
Mn2£«£« ClO
ClO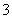 £«
£« H2O===
H2O=== MnO2”ż£«
MnO2”ż£« Cl2”ü£«
Cl2”ü£« ________”£
________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
5.6 g Cu”¢MgŗĻ½šÓėŅ»¶ØĮæµÄĻõĖįĒ”ŗĆĶźČ«·“Ó¦£¬ŹÕ¼Æµ½NOŗĶNO2µÄ»ģŗĻĘųĢåV L(±ź×¼×“æö)£»Ļņ·“Ó¦ŗóµÄČÜŅŗÖŠ¼ÓČė×ćĮæNaOHČÜŅŗ£¬³ĮµķĶźČ«ŗó½«Ęä¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬³ĘµĆÖŹĮæĪŖ10.7 g”£ŌņVµČÓŚ(””””)
A£®2.24 B£®4.48
C£®6.72 D£®7.84
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NO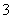 (H£«)²»ÄÜŗĶ______________________________________“óĮæ¹²“ę”£
(H£«)²»ÄÜŗĶ______________________________________“óĮæ¹²“ę”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Š“³öĻĀĮŠµäŠĶĪļÖŹµÄµēĄė·½³ĢŹ½
(1)H2SO4______________________________________________________________________£»
(2)H2CO3______________________________________________________________________£»
(3)Ca(OH)2____________________________________________________________________£»
(4)Fe(OH)3_____________________________________________________________________£»
(5)NH3·H2O____________________________________________________________________£»
(6)NaCl_______________________________________________________________________£»
(7)BaSO4_____________________________________________________________________£»
(8)NaHSO4_____________________________________________________________________£»
(9)NaHCO3____________________________________________________________________£»
(10)NaHSO4(ČŪČŚ)_____________________________________________________________£»
(11)Al2O3(ČŪČŚ)___________________________________________________________£»
(12)CH3COOH_______________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com