
��10�֣�ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
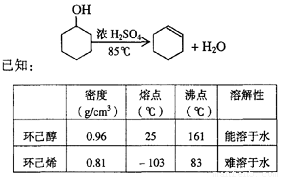
��1���Ʊ���Ʒ��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��[��Դ:Z+xx+k.Com]
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
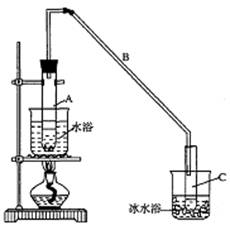
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��룬Ŀ����__________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
a������ʱ��70 �濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
(1)�� ��ֹ����(����������Ҳ����) ����
�� ��ֹ����ϩ�Ļӷ�(����������Ҳ����)
(2) ���� c ��g ��ȴˮ�������γ����� ��83�� c���� (3)b c
���ֱ���(3)2�֣�ѡ��һ����һ�֣���ѡû�֣�����cѡ��2�֣�����ÿ��һ��
����������1����Ϊ��Ӧ��Ҫ���ȣ�Ϊ�˷�ֹҺ�����ʱ������������Ҫ���Ƭ��ֹ���С�ʵ������Ҫ��ʱ�ѻ���ϩ��������������B���������������á�����ʱ���������ӷ���Ϊ�����ԭ�ϵ������ʣ���Ҫͨ��ˮԡ���ȷ�ֹ�������ӷ���
��2������ϩ���ܶ�С��ˮ��������ˮ�����ϲ㡣���Ը��������Һ����ǿ�����ԣ�����������ϩ��ϡ���������ԣ����ܳ�ȥ�������ʣ������ñ���̼������Һϴ�ӡ�����������ȴˮ�������������������෴�ģ����Դ�g�ڽ��롣����ϩ�ķе���83�棬�����¶�Ӧ������83�����ҡ�����ϩ�ľ�Ʒ����ƫ�ͣ�˵���ֲ�Ʒ�����������ʶ࣬���Կ������Ʊ��ֲ�Ʒʱ������һͬ���������ֲ�Ʒ�;�Ʒ���������ڴֲ�Ʒ�к��л�����������Ʒ��û�У��ƺʹ���Ӧ��b���ԡ��������ķе�ͻ���ϩ�ķе���ϴ�cҲ���ԡ����Ը��������Һ����ǿ�����ԣ��������������ͻ���ϩ������ȷ����ѡbc��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС���������������������װ�ã���ͼ��)�Ի������Ʊ�����ϩ��

��֪��
| �ܶȣ�g��cm-3) | �۵㣨��)�� | �㣨��) | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

ͼ��
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������_____________������B���˵�������е�������_______________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_________________________________________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ��кͱ���ʳ��ˮ�������á��ֲ㣬����ϩ��_______________�㣨��ϡ����¡�������Һ����_______________�������ţ�ϴ�ӡ�
a.KMnO4��Һ
b.ϡH2SO4
c.Na2CO3��Һ

ͼ��
���ٽ�����ϩ��ͼ��װ��������ȴˮ��____________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����____________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��____________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________��
a.����ʱ��70 �濪ʼ�ռ���Ʒ
b.������ʵ����������
c.�Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������____________��
a.�ø������������Һ b.�ý����� c.�ⶨ�е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ɽһ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(11��)ij��ѧС���������������������װ��(��ͼ)���Ի������Ʊ�����ϩ��
��֪��

| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ÷������������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��20�֣�ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____ ��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________________________��
��2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��______��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����______________ ____��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����____________________
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� C���ⶨ�е�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com