
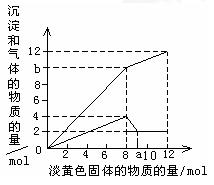

| ���ܺ��е����� �������ӷ��ţ� | | | | | |
| ��Ӧ�����ʵ��� | | | | | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��2F2+2H2O=4HF+O2 | B��2Na+2H2O=2NaOH+H2�� |
| C��CaO+H2O=Ca(OH)2 | D��2H2O=2H2��+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ĽṹʽΪ��H��Cl��O | B����OH �� ����ʾ�ǻ� ����ʾ�ǻ� |
C���Ȼ�淋ĵ���ʽ�� | D������ķ��ӱ���ģ��Ϊ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH=3�������pH=11��Ba(OH)2��Һ | B��pH=3�������pH=11�İ�ˮ |
| C��pH=3�������pH=11��KOH | D��pH=3�Ĵ����pH=11��KOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Na2CO3������Һ��ͨ��CO2����NaHCO3�ᾧ���� |
| B����ҵ����ȡ���ʹ� |
| C��Na2O2����������еĹ����� |
| D��һ��������������ͭ��CuH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H2+Cl2 2HCl 2HCl | B��CaCO3+2HCl=CaCl2+CO2 ��+H2O |
C��CuSO4+BaCl2=BaSO4 ��+CuCl2 ��+CuCl2 | D��NH4HCO3 NH3��+CO2��+H2O NH3��+CO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ������ķ���ʽC2H5Cl | B���Ҵ��Ľṹ��ʽC2H6O |
C��F��ԭ�ӽṹʾ��ͼ��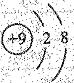 | D�����Ȼ�̼�ĵ���ʽ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com