
£Ø1£©Š“³öÉĻŹö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½______________£¬ŹµŃéŹŅÖŠ¼ų±šŅŅČ²ŗĶ¼×ĶéµÄ×ī¼ņµ„µÄ·½·ØŹĒ_________________”£ĻąĶ¬ÖŹĮæµÄŅŅČ²ŗĶ¼×ĶéČ¼ÉÕŹ±Éś³ÉĖ®¶ąµÄŹĒ__________________”£
£Ø2£©ÓÉÓŚ±½µÄŗ¬Ģ¼ĮæÓėŅŅČ²ĻąĶ¬£¬æŖŹ¼ČĖĆĒČĻĪŖĖüĆĒŹĒŅ»ÖÖ²»±„ŗĶµÄĢž£¬Š“³öC 6H 6µÄŅ»ÖÖŗ¬Čž¼üĒŅĪŽ¼×»łµÄĮ“ĢžµÄ½į¹¹¼ņŹ½_______________£¬ÄÜĖµĆ÷±½µÄ½į¹¹²»ŹĒµ„”¢Ė«¼ü½»ĢęµÄÕżĮł±ßŠĪĘ½Ćę½į¹¹µÄŹĀŹµŹĒ______________________”£?
£Ø3£©ĻĀĮŠ¹ŲÓŚ±½ŗĶĪŽ»ś±½µÄŠšŹö²»ÕżČ·µÄŹĒ”£?
A£®±½·Ö×ÓÖŠø÷Ō×Ó¶¼ŌŚĶ¬Ņ»Ę½ĆęÉĻ£¬ĪŽ»ś±½ÖŠø÷Ō×Ó²»ŌŚĶ¬Ņ»Ę½ĆęÉĻ?
B£®ĪŽ»ś±½ŌŚŅ»¶ØĢõ¼žĻĀæÉ·¢ÉśČ”“ś·“Ó¦ŗĶ¼Ó³É·“Ó¦?
C£®Óė±½ĻąĮŚµÄ±½µÄĶ¬ĻµĪļµÄŅ»ĀČ“śĪļÓŠ4ÖÖ£¬ĪŽ»ś±½µÄ¶žĀČ“śĪļÓŠ4ÖÖ?
D£®ĪŽ»ś±½²»ÄÜŹ¹ĖįŠŌKMnO 4ČÜŅŗĶŹÉ«?
E£®¼ų±š»·ŅŅČ²ŗĶ±½æÉŅŌ½«äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·Ö±šµĪ¼Óµ½ÉŁĮæ»·ŅŅĻ©ŗĶ±½ÖŠ?
£Ø1£©CaCl2+2H2O![]() Ca(OH)2+C2H2”ü””µćČ¼¹Ū²ģŹĒ·ńĆ°ÅصÄŗŚŃĢ””¼×Ķé?
Ca(OH)2+C2H2”ü””µćČ¼¹Ū²ģŹĒ·ńĆ°ÅصÄŗŚŃĢ””¼×Ķé?
£Ø2£©![]() ±½µÄĮŚ¶žČ”“śĪļƻӊĶ¬·ÖŅģ¹¹Ģå?
±½µÄĮŚ¶žČ”“śĪļƻӊĶ¬·ÖŅģ¹¹Ģå?
£Ø3£©A
½āĪö£ŗ£Ø1£©·“Ó¦ĪŖCaC2+2H2O![]() Ca(OH)2+C2H2”ü£¬¼ų±šŅŅČ²ŗĶ¼×ĶéÓĆČ¼ÉÕ£¬¼×ĶéÓÉÓŚŗ¬ĒāĮæøߣ¬¹ŹĶ¬ÖŹĮæČ¼ÉÕ¼×ĶéÉś³ÉĖ®¶ą”£?
Ca(OH)2+C2H2”ü£¬¼ų±šŅŅČ²ŗĶ¼×ĶéÓĆČ¼ÉÕ£¬¼×ĶéÓÉÓŚŗ¬ĒāĮæøߣ¬¹ŹĶ¬ÖŹĮæČ¼ÉÕ¼×ĶéÉś³ÉĖ®¶ą”£?
£Ø2£©Ó¦ÓŠĮ½øö![]() £¬¹ŹĪŖ
£¬¹ŹĪŖ![]() £¬ÄÜÖ¤Ć÷²»ŹĒµ„”¢Ė«¼ü½»ĢęµÄŹĒ±½µÄĮŚ¶žČ”“śĪļÖ»ÓŠŅ»ÖÖ”£?
£¬ÄÜÖ¤Ć÷²»ŹĒµ„”¢Ė«¼ü½»ĢęµÄŹĒ±½µÄĮŚ¶žČ”“śĪļÖ»ÓŠŅ»ÖÖ”£?
£Ø3£©Óɱ½ŠŌÖŹĶĘÖŖA²»ÕżČ·”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| Fe |
| Fe |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ”÷ |



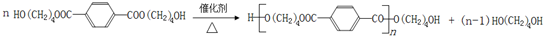
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŌĘÄĻÓńĻŖŅ»ÖŠø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌĄķæĘ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
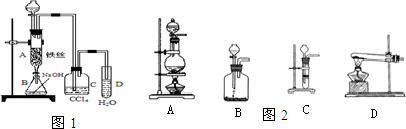
£Ø15·Ö£©£Ø1£©Ä³»ÆѧæĪĶāŠ”×éÓĆÓŅĶ¼×°ÖĆÖĘČ”äå±½”£ĻČĻņ·ÖŅŗĀ©¶·ÖŠ¼ÓČė±½ŗĶŅŗä壬ŌŁ½«»ģŗĻŅŗĀżĀżµĪČė·“Ó¦Ę÷A£ØAĻĀ¶Ė»īČū¹Ų±Õ£©ÖŠ”£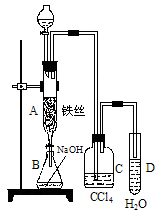
¢ŁŠ“³öAÖŠÓŠ»ś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
¢ŚŅŃÖŖÉĻŹöÓŠ»ś·“Ó¦ŹĒ·ÅČČ·“Ó¦£¬¹Ū²ģµ½AÖŠµÄĻÖĻóŹĒ£ŗ
¼°_____ _________”£
¢Ū ŹµŃé½įŹųŹ±£¬“ņæŖAĻĀ¶ĖµÄ»īČū£¬ČĆ·“Ó¦ŅŗĮ÷ČėBÖŠ£¬³ä·ÖÕńµ“£¬ÄæµÄŹĒ £¬Š“³öÓŠ¹ŲµÄ»Æѧ·½³ĢŹ½ ”£
¢ÜCÖŠŹ¢·ÅCCl4µÄ×÷ÓĆŹĒ ”£
¢ŻÄÜÖ¤Ć÷±½ŗĶŅŗäå·¢ÉśµÄŹĒČ”“ś·“Ó¦£¬¶ų²»ŹĒ¼Ó³É·“Ó¦£¬æÉĻņŹŌ¹ÜDÖŠµĪČėAgNO3ČÜŅŗ£¬Čō²śÉśµ»ĘÉ«³Įµķ£¬ŌņÄÜÖ¤Ć÷”£ĮķŅ»ÖÖŃéÖ¤µÄ·½·ØŹĒĻņŹŌ¹ÜDÖŠ¼ÓČė______ _____£¬ĻÖĻóŹĒ______________________”£
£Ø2£©ŅŅČ²µÄŹµŃéŹŅÖĘ·Ø
¢Ł·“Ó¦ŌĄķ_____ ____________”£
¢ŚŃ”ŌńŗĻŹŹµÄÖĘČ”ŹµŃé×°ÖĆ___ ___”£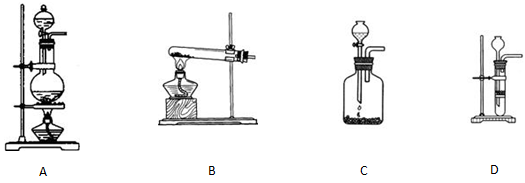
¢ŪŹµŃéÖŠ³£ÓƱ„ŗĶŹ³ŃĪĖ®“śĢęĖ®£¬ÄæµÄŹĒ______ __________”£
¢Ü“æ¾»µÄŅŅČ²ĘųĢåŹĒĪŽÉ«ĪŽĪ¶µÄĘųĢ壬ÓƵēŹÆŗĶĖ®·“Ó¦ÖĘČ”µÄŅŅČ²£¬³£ŗ¬ÓŠH2SŗĶPH3¶ųÓŠ¶ń³ōĘųĪ¶”£æÉŅŌÓĆ____ _______ČÜŅŗ³żČ„ŌÓÖŹĘųĢ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÖŲĒģŹŠø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĻĀĮŠŹµŃéÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ_____________”£
A£®ÓĆĮņĖįĶČÜŅŗ³żČ„µēŹÆÓėĖ®·“Ӧɜ³ÉµÄŅŅČ²ĘųĢåÖŠµÄŌÓÖŹ
B£®äåŅŅĶéÓėNaOHĖ®ČÜŅŗ»ģŗĻ¹²ČČŅ»¶ĪŹ±¼äŗó£¬ĻņĖ®²ć¼ÓČėAgNO3ČÜŅŗ£¬¹Ū²ģµ½µ»ĘÉ«³Įµķ²śÉś”£
C£®¼×±½ÓėŅŗäå»ģŗĻ£¬¼ÓČėÉŁĮæĢśŠ¼£¬Öʱø ”£
ӣ
D£®ŅŃÖŖ3.0 mol”¤L£1 CuSO4ČÜŅŗÖŠCu2+æŖŹ¼³ĮµķµÄpHĪŖ4£¬Fe3+ĶźČ«³ĮµķŹ±ČÜŅŗµÄpHĪŖ3.3£¬ŌņæÉŅŌÓĆŃõ»ÆĶ³żČ„øĆČÜŅŗÖŠŗ¬ÓŠµÄŌÓÖŹFe3+
E£®Ö»ÓĆFeCl3ČÜŅŗ¾ĶæÉŅŌ¼ų±š³ö±½·ÓČÜŅŗ”¢NaOHČÜŅŗ”¢KHCO3ČÜŅŗ”¢±½”¢äåŅŅĶéĪåÖÖĪŽÉ«ŅŗĢå
F£®ÓĆĻ”ŃĪĖįŗĶäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗæÉÖ¤Ć÷1-äåĪģĶéŌŚNaOHĖ®ČÜŅŗÓėNaOH“¼ČÜŅŗÖŠ·¢ÉśµÄ·“Ó¦ĄąŠĶ²»Ķ¬
G£®ĶعżŹÆÓĶµÄ·ÖĮóæÉŅŌÖ±½ÓµĆµ½¼×Ķ锢ŅŅĻ©ŗĶ±½µČ²śĘ·
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com