

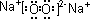 ��BΪCO2���ɽṹʽO=C=O���Կ�����ӦΪ���м��Լ��ķǼ��Է��ӣ��ʴ�Ϊ��
��BΪCO2���ɽṹʽO=C=O���Կ�����ӦΪ���м��Լ��ķǼ��Է��ӣ��ʴ�Ϊ�� ���Ǽ��ԣ�
���Ǽ��ԣ� =0.5mol��
=0.5mol�� =5.6L��
=5.6L��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���2011��ģ�⣩��ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ��
�ش��������⣺
��1��A�ĵ���ʽ ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ���һ�и�����һ���¿���ѧ�Ծ� ���ͣ������
��12�֣���2011��ģ�⣩��ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ��
�ش��������⣺
��1��A�ĵ���ʽ ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����һ�и����ڶ����¿����ۺϲ��ԣ���ѧ���֣� ���ͣ������
��16�֣���ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ�����治�����ʻ�ɫ��
�ش��������⣺
��1��A�ĵ���ʽΪ ��B�������� ���ӣ����ԡ��Ǽ��ԣ���
��2����Ӧ�ٵĻ�ѧ����ʽΪ ��
��Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
��4������K�������ӳ��õķ����� ��
��5��LŨ��Һ�ı��淽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ������ѧ���ڳ����Ի�ѧ�Ծ� ���ͣ������
��12�֣���ͼ����ѧ�������ʼ��ת����ϵ����֪��
��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ� ��EΪ����������JΪ���ɫ������
��G��ʵ�����г����ڼ���B�Ĵ��ڣ���L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ����������ɫƿ�С�
�ش��������⣺
��1��A�ĵ���ʽ____________________________________________________��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
��4��L�Ļ�ѧʽ ��G�Ļ�ѧʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com