
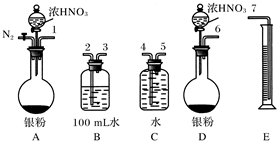
ij����С�����������ʵ�鷽����֤Ag��ŨHNO3��Ӧ�Ĺ����п��ܲ���NO����ʵ������ͼ���£�
�Ųⶨ��������ʵ���
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol��L��1��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ____________����Ag��Ũ���ᷴӦ���������ɵ�NO2�����ʵ���Ϊ______________��
�ƲⶨNO�����
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������____________________________________________________________
________________________________________________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����_________________________(������ܿڱ��)��
���ڲⶨNO�����ʱ������Ͳ��ˮ��Һ��ȼ���ƿ��Һ��Ҫ�ͣ���ʱӦ����Ͳ��λ��______(ѡ��½��������ߡ�)���Ա�֤��Ͳ�е�Һ���뼯��ƿ�е�Һ���ƽ��
������ɷַ���
��ʵ����NO�����Ϊ112.0mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO�����������жϵ�������__________________
________________________________________________________________________��
(14��)��0.008mol ��2�֣� 0.012 mol ��1�֣�
Bװ���з����ķ�Ӧ��3NO2��H2O===2HNO3��NO����NaOH��Һ�ζ�����HNO3����n(HNO3)��4n(NaOH)��4��0.1 mol��L��1��(20.40��0.40)��10��3 L��0.008 mol��������NO2�����ʵ���Ϊ��0.008 mol����0.012 mol��
�Ƣ�A��2�֣�
��ΪAװ�ÿ���ͨ��N2��װ���еĿ����ž�����ֹNO��������O2������2�֣�
��123547��2�֣���
������ ��1�֣�
���У�2�֣�
��ΪNO2��ˮ��Ӧ���ɵ�NO�����С���ռ�����NO�����(89.6<112.0) ��2�֣�
����:��


 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
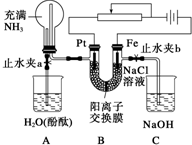 ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2 | 3 |
�鿴�𰸺ͽ���>>
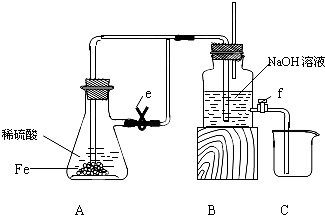
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ϡ���� |
| �� |
| NaOH��Һ |
| ��ˮ |
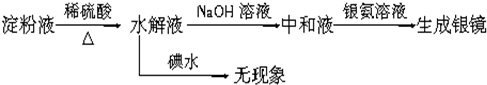
| ϡ���� |
| �� |
| ������Һ |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com