
| ����ǰ���� | ����ǰ���� | ���Ⱥ����� |
| W1�������� | W2������+���壩 | W3������+��ˮ����ͭ�� |
| 160(W2-W3) |
| 18(W3-W1) |
| 160(W2-W3) |
| 18(W3-W1) |
| 0.418��2.35 |
| 0.025 |
| 0.418��2.35 |
| 0.025 |
| Q |
| n |
| 160(W2-W3) |
| 18(W3-W1) |
| 160(W2-W3) |
| 18(W3-W1) |
| 2.3��+2.4�� |
| 2 |
| 0.418��2.35kJ |
| 0.025mol |
| 0.418��2.35 |
| 0.025 |
| 0.418��2.35 |
| 0.025 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.���� B.���� C.���� D.�ؽᾧ
E.���� F.���� G.��� H.���ȷֽ�
I.����
���и�������ķ�����ᴿӦѡ����һ�ַ�������ʣ�
(1)��ȥCa(OH)2��Һ��������CaCO3������___________��
(2)��ȥFe(OH)3�����л��е�Cl-���ӣ���___________��
(3)��ȥ�Ҵ����ܽ����ʳ�Σ���___________��
(4)����ʯ���и��ֲ�ͬ�е㷶Χ�ijɷ֣���___________��
(5)��ȥ������л��е�����NaI����___________��
(6)��ȥ�������е�CaCO3___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
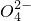 ��Cl-������______��
��Cl-������______��| ����ǰ���� | ����ǰ���� | ���Ⱥ����� |
| W1�������� | W2������+���壩 | W3������+��ˮ����ͭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com