
ij��ѧѧϰС�飬��̽��CO2��NaOH�Ƿ�����Ӧʱ����Ƴ���������װ�ý���ʵ�飺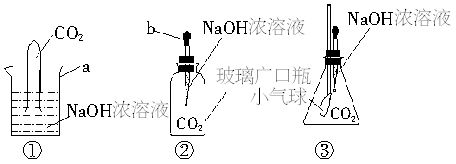
��ش����¼������⣺
��1��д����ͼ�б�����ĸ���������ƣ�a______��b______��
��2����������ʵ���У��٢��������������������С��ͬѧ��¼���ǹ۲쵽��ʵ������
ʵ���__________________________________��
ʵ���__________________________________��
��3��ʵ�����ѡ��������������δ�ܹ۲쵽���������������ѧ����֪ʶ��Ѱ��һ�������еij�����Ʒ�����װ���еĹ��ƿ����ʹʵ��ȡ�óɹ����㽫ѡ�õ���Ʒ��______���Ľ����ܿ�����ʵ��������_________________________________��
��4�������һ�����ʵ��������CO2��NaOH��Һ��Ӧ������Na2CO3�������йص�ʵ������������������±���
��5�����鷴Ӧ������Na2CO3�����ӷ���ʽΪ____________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ�г���ʯ��ˮ�� 8.0mol?L-1 8.0mol?L-1 NaOH��Һ NaOH��Һ ��ϴ��ƿ�У����������ǵ�ľ���������һ��ϴ��ƿ�ij��ڴ� ���������ǵ�ľ���������һ��ϴ��ƿ�ij��ڴ� �� |
������ʯ��ˮ������ǣ�ľ����ȼ�������1������ �� ����ʯ��ˮ����ǣ�ľ����ȼ ����ʯ��ˮ����ǣ�ľ����ȼ �������2������������ʯ��ˮ����ǣ�ľ������ȼ�������3������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ��У�����ڶ���������ѧ�Ծ��������棩 ���ͣ�ʵ����
��̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��
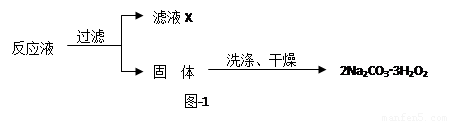
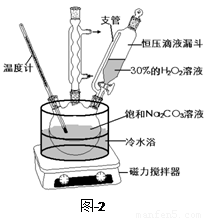
��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3��������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ��÷���ʽ��ʾ����________________________________��
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0��1mol/LNaOH��Һ��8��0mol/LNaOH��Һ������ʯ��ˮ��0��01mol/LKMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������______________�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
|
ʵ�鲽�� |
Ԥ����������� |
|
����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� |
��________________________ ��________________________ ��________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ�г���ʯ��ˮ��______ ______��ϴ��ƿ�У�______�� |
������ʯ��ˮ������ǣ�ľ����ȼ�������1������ ��______�������2������ ������ʯ��ˮ����ǣ�ľ������ȼ�������3������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ�ʵ����
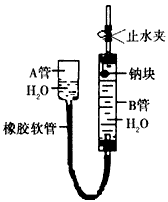
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com