
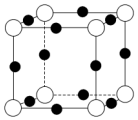
 ��֪��A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�أ�����Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�Fԭ�Ӻ���������������B��ͬ���������������������������Ϣ���ش��������⣺
��֪��A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�أ�����Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�Fԭ�Ӻ���������������B��ͬ���������������������������Ϣ���ش��������⣺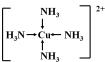 ���ü�ͷ��ʾ���ṩ�¶Ե��ӵ�ԭ�ӣ����� F ���ʵķ�ĩ����A����̬�⻯���ˮ����Ũ��Һ�У�ͨ�� O2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽΪ2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
���ü�ͷ��ʾ���ṩ�¶Ե��ӵ�ԭ�ӣ����� F ���ʵķ�ĩ����A����̬�⻯���ˮ����Ũ��Һ�У�ͨ�� O2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽΪ2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O������ A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�أ�Aԭ�Ӻ���������δ�ɶԵ��ӣ�������Ų�ʽΪ1s22s22p3��ΪNԪ�أ�Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p63s23p4��ӦΪSԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�ΪAlԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬BӦΪ�ڢ�A��Ԫ�أ���ԭ��������NԪ�غ�Al֮�䣬ӦΪNaԪ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�ӦΪSiԪ�أ����ʹ�Ϊԭ�Ӿ��壬�۵��ڵ�����������ߣ�Fԭ�Ӻ���������������B��ͬ������������������ԭ������������Ų�ʽΪ1s22s22p63s23p63d104s1��ӦΪCuԪ�أ��ݴ˽��н��
��� �⣺A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�أ�Aԭ�Ӻ���������δ�ɶԵ��ӣ�������Ų�ʽΪ1s22s22p3��ΪNԪ�أ�Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p63s23p4��ӦΪSԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�ΪAlԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬BӦΪ�ڢ�A��Ԫ�أ���ԭ��������NԪ�غ�Al֮�䣬ӦΪNaԪ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�ӦΪSiԪ�أ����ʹ�Ϊԭ�Ӿ��壬�۵��ڵ�����������ߣ�Fԭ�Ӻ���������������B��ͬ������������������ԭ������������Ų�ʽΪ1s22s22p63s23p63d104s1��ӦΪCuԪ�أ�
��1����NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壬ԭ�Ӿ�����۷е�Զ���ڷ��Ӿ�����۷е㣬
�ʴ�Ϊ���ߣ�NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壻
��2��E��S����D��Si����ͬ�������Ԫ�ؿ��γ�һ�ֻ�����X��XΪCS2��������������к�������˫����Ϊֱ���ͽṹ��
�ʴ�Ϊ��ֱ���ͣ�
��3��FCuԪ�أ�ԭ������Ϊ29����������Ų�ʽΪ��1s22s22p63s23p63d104s1��ͭ���ӺͰ��������γ�4����λ�����γ�ͭ���������Ϊ[Cu��NH3��4]2+����ṹʽΪ�� ��
��
��Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ��˵���õ��İ���ͭ�����ӣ���Ӧ����������������ˮ���÷�Ӧ�����ӷ���ʽΪ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
�ʴ�Ϊ��1s22s22p63s23p63d104s1�� ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
��4��A��NԪ�ء�F��CuԪ�أ�NԪ�ػ��ϼ�Ϊ-3�ۣ��þ����а�ɫ�����=8��$\frac{1}{8}$=1����ɫ�����=12��$\frac{1}{4}$=3�����ݻ��ϼ�֪����ɫ���ʾNԭ�ӡ���ɫ���ʾCuԭ�ӣ��仯ѧʽΪCu3N��
�ʴ�Ϊ��Cu3N��
��5��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ�����ԭ�Ӿ��壬�仯�����������Ļ�ѧ������Ϊ���ۼ���
�ʴ�Ϊ�����ۼ���
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢���ռ��ϵ�жϡ�����ԭ�Ӻ�������Ų���֪ʶ�㣬�ۺ��Խ�ǿ�����ؿ���ѧ�����㡢�жϼ�֪ʶ�ۺ��������������ջ���֪ʶ����������ǽⱾ��ؼ����ѵ����ռ乹���жϼ��������㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ˮ�ֽ��Ƶ�������һ�ֹ�ҵ�����ǡ���-��ѭ�������������漰����������Ӧ��
��ˮ�ֽ��Ƶ�������һ�ֹ�ҵ�����ǡ���-��ѭ�������������漰����������Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ��ˮ | B�� | NaOH��Һ | C�� | ���� | D�� | CO2��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
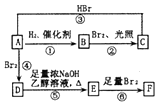 ϩ��A��һ�������¿����¶������̽��з�Ӧ��
ϩ��A��һ�������¿����¶������̽��з�Ӧ��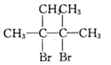
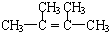 ��
��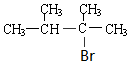 ��
��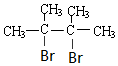 +2NaOH$��_{��}^{�Ҵ�}$
+2NaOH$��_{��}^{�Ҵ�}$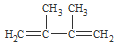 +2NaBr+2H2O��
+2NaBr+2H2O��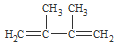 +2Br2��
+2Br2��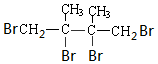 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
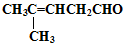 ���������ȩ�������õ��Լ���������Һ�������Ʊ�������ͭ��Һ������ѧ����ʽΪ
���������ȩ�������õ��Լ���������Һ�������Ʊ�������ͭ��Һ������ѧ����ʽΪ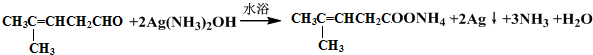 ��
��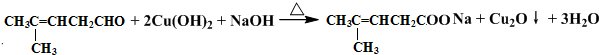 ��
��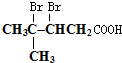 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʳƷ�м�����ɫ�� | B�� | �ִ�����װп����� | ||
| C�� | �ڸ���װ�ڷ��ÿ������� | D�� | ȼú����ʱ��ú�۴���ú�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ij��Һ�м���ϡ�����ữ���ٵ���BaCl2��Һ��������ɫ��������ԭ��Һ��һ����SO42- | |
| B�� | ��ij��Һ�м���AgNO3��Һ��������ɫ��������ԭ��Һ��һ����Cl-? | |
| C�� | �ù��IJ�˿պȡij��ɫ��Һ���ھƾ�������������ʱ�۲쵽��ɫ���棬��ԭ��Һ��һ����Na+ | |
| D�� | ��ij��Һ�м���̼������Һ��������ɫ�������ٵ���ϡ���ᣬ�����ܽ⣬��ԭ��Һ��һ����Ca2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
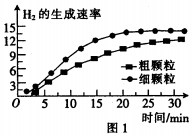

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
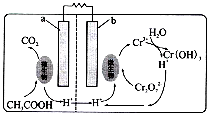
| A�� | b������ | |
| B�� | a����Ӧʽ��CH3COOH+2H2O-8e-=2CO2+8H+ | |
| C�� | ÿ����1mol Cr2O72-����CO2����״���£�3.36 L | |
| D�� | ÿ����lmol Cr��OH��3���ҳ�n��H+������2 mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com