
| A£® | 0.4% | B£® | 0.7% | C£® | 1% | D£® | 1.3% |
·ÖĪö Ī²ĘųĶØČėĒāŃõ»ÆÄĘ·“Ó¦Ź±£¬NO2ŗĶNOĒ”ŗƱ»ĶźČ«ĪüŹÕ£¬·¢Éś·“Ó¦£ŗNO2+NO+2NaOH=2NaNO2+H2O£¬2NO2+2NaOH=NaNO2+NaNO3+H2O£¬ÓÉŌŖĖŲŹŲŗćæÉÖŖ£ŗÄĘŌ×ÓÓėµŖŌ×ÓĪļÖŹµÄĮæĻąµČ£¬¼“n£ØNO2+NO£©=n£ØNaOH£©£¬¾Ż“Ė¼ĘĖć»ģŗĻĘųĢåÖŠV£ØNO2+NO£©£»
Ī²ĘųĶعż“ß»Æ×Ŗ»»Ę÷Ź±£¬·¢Éś·“Ó¦£ŗ4CO+2NO2ØTN2+4CO2£¬2CO+2NOØTN2+2CO2£¬ÓÉ·½³ĢŹ½æÉÖŖ£¬Č«²æĪŖNO2Ź±£¬COµÄĢå»ż×ī“ó£¬Č«²æĪŖNOŹ±£¬COµÄĢå»ż×īŠ”£¬½ų¶ų¼ĘĖćCOĢå»ż£¬ŌŁ¼ĘĖćCOĢå»ż·ÖŹż·¶Ī§£®
½ā“š ½ā£ŗ·¢Éś·“Ó¦£ŗ4CO+2NO2ØTN2+4CO2”¢2CO+2NOØTN2+2CO2£¬
Ī²ĘųĶØČėĒāŃõ»ÆÄĘ·“Ó¦Ź±£¬NO2ŗĶNOĒ”ŗƱ»ĶźČ«ĪüŹÕ£¬·¢Éś·“Ó¦NO2+NO+2NaOH=2NaNO2+H2O”¢2NO2+2NaOH=NaNO2+NaNO3+H2O£¬ÓÉŌŖĖŲŹŲŗćæÉÖŖ£ŗÄĘŌ×ÓÓėµŖŌ×ÓĪļÖŹµÄĮæĻąµČ£¬¼“n£ØNO2+NO£©=n£ØNaOH£©=0.05L”Į0.1mol/L=0.005mol£¬¹ŹV£ØNO2+NO£©=0.005L”Į22.4L/mol=0.112L£¬
Ī²ĘųĶعż“ß»Æ×Ŗ»»Ę÷Ź±£¬·¢Éś·“Ó¦£ŗ4CO+2NO2ØTN2+4CO2£¬2CO+2NOØTN2+2CO2£¬ÓÉ·½³ĢŹ½æÉÖŖ£ŗ
Č«²æĪŖNO2Ź±£¬COµÄĢå»ż×ī“ó£¬COĢå»ż×ī“óĪŖ0.112L”Į2=0.224L£¬¹ŹCOĢå»ż·ÖŹż×ī“óÖµĪŖ£ŗ$\frac{0.224L}{22.4L}$”Į100%=1%£»
Č«²æĪŖNOŹ±£¬COµÄĢå»ż×īŠ”£¬COĢå»ż×īŠ”ĪŖ0.112L£¬¹ŹCOĢå»ż·ÖŹż×īŠ”ÖµĪŖ£ŗ$\frac{0.112L}{22.4L}$”Į100%=0.5%£»
¹ŹCOĢå»ż·ÖŹż·¶Ī§½éÓŚ0.5%”«1%Ö®¼ä£¬
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²é»ģŗĻĪļ¼ĘĖć£¬ĢāÄæÄѶČÖŠµČ£¬¹Ų¼üŹĒĄūÓĆŌ×ÓŹŲŗć¼ĘĖ涞Ńõ»ÆµŖÓėNO×ÜĢå»ż£¬ŌŁĄūÓĆ¼«ĻŽ·Ø½ųŠŠ·ÖĪö½ā“š£¬²ąÖŲæ¼²éѧɜ·ÖĪöÄÜĮ¦¼°»Æѧ¼ĘĖćÄÜĮ¦£®


 ø÷µŲĘŚÄ©ø“Ļ°ĢŲѵ¾ķĻµĮŠ“š°ø
ø÷µŲĘŚÄ©ø“Ļ°ĢŲѵ¾ķĻµĮŠ“š°ø Š”²©ŹæĘŚÄ©“³¹Ų100·ÖĻµĮŠ“š°ø
Š”²©ŹæĘŚÄ©“³¹Ų100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ī÷¹ĻŹĒĖįŠŌŹ³Īļ | B£® | ÖķČā”¢Å£ČāŹĒ¼īŠŌŹ³Īļ | ||
| C£® | öĻÓć”¢ÄĢÓĶŹĒĖįŠŌŹ³Īļ | D£® | “óĆ×”¢Ćę°üŹĒ¼īŠŌŹ³Īļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 0.75 mol/L | B£® | 0.25 mol/L | C£® | 0.5 mol/L | D£® | 1 mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | KClO3ŗĶSO3ČÜÓŚĖ®ŗóÄܵ¼µē£¬¹ŹKClO3ŗĶSO3ĪŖµē½āÖŹ | |
| B£® | Na2SO3ČÜŅŗÖŠ£ŗc£ØH+£©+c£ØHSO3-£©+c£ØH2SO3£©ØTc£ØOH-£© | |
| C£® | ĻņNaAlO2ČÜŅŗÖŠµĪ¼ÓNaHCO3ČÜŅŗ£¬ÓŠ³ĮµķŗĶĘųĢåÉś³É | |
| D£® | Ļņŗ¬ÓŠAgCl¹ĢĢåµÄČÜŅŗÖŠ¼ÓČėŹŹĮæĖ®Ź¹AgClČܽāÓÖ“ļµ½Ę½ŗāŹ±£¬ŌŚøĆĪĀ¶ČĻĀAgClµÄČܶȻż²»±ä£¬ĘäČܽā¶ČŅ²²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N2 | B£® | NH3 | C£® | HCl | D£® | SO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
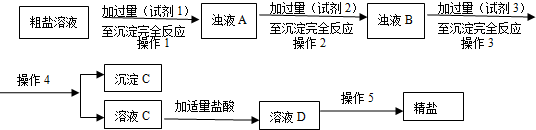
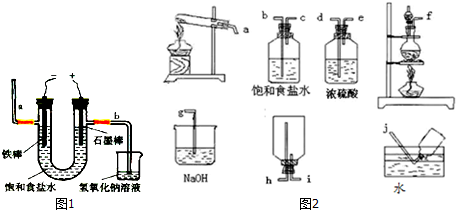
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{22.4N}{V}$ mol-1 | B£® | $\frac{VN}{22.4}$mol-1 | C£® | $\frac{VN}{11.2}$ mol-1 | D£® | $\frac{11.2N}{V}$ mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ŌŚ2LĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦3Fe£Øs£©+4H2O£Øg£©?Fe3O4£Øs£©+4H2£Øg£©”÷H£¾0²āµĆn£ØH2O£©Ėę·“Ó¦Ź±¼ä£Øt£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ£Ø””””£©
ŌŚ2LĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦3Fe£Øs£©+4H2O£Øg£©?Fe3O4£Øs£©+4H2£Øg£©”÷H£¾0²āµĆn£ØH2O£©Ėę·“Ó¦Ź±¼ä£Øt£©µÄ±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ£Ø””””£©| A£® | øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖK=c4£ØH2O£©/c4£ØH2£© | |
| B£® | 5minŹ±øĆ·“Ó¦µÄ¦Ō£ØÕż£©µČÓŚ9minŹ±µÄ¦Ō£ØÄę£© | |
| C£® | 0”«5minÄŚ£¬¦Ō£ØH2£©=0.10mol/£ØL•min£© | |
| D£® | 10minŹ±Ę½ŗā·¢ÉśŅʶÆæÉÄÜŹĒĶ¶Čė»¹ŌŠŌĢś·ŪŅżĘš |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | FeµÄÖŹĮæĪŖ2.7g£¬AlµÄÖŹĮæĪŖ2.8g | B£® | FeµÄÖŹĮæĪŖ2.8g£¬AlµÄÖŹĮæĪŖ2.7g | ||
| C£® | FeµÄÖŹĮæĪŖ5.4g£¬AlµÄÖŹĮæĪŖ5.6g | D£® | FeµÄÖŹĮæĪŖ5.6g£¬AlµÄÖŹĮæĪŖ5.4g |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com