
���������з�Ӧ�ϳ������谷��CaO��3C CaC2��CO����CaC2��N2
CaC2��CO����CaC2��N2 CaCN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
CaCN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
(1)д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ��_____________________________________________��
CaCN2��������ΪCN ����CN
����CN ��Ϊ�ȵ�����ķ�����N2O��________(�ѧʽ)���ɴ˿�����֪CN
��Ϊ�ȵ�����ķ�����N2O��________(�ѧʽ)���ɴ˿�����֪CN �Ŀռ乹��Ϊ________��
�Ŀռ乹��Ϊ________��
(2)���ط�����Cԭ�Ӳ�ȡ________�ӻ������ط��ӵĽṹ��ʽ��________��
 (3)�����谷(
(3)�����谷( )�׳ơ����������������������谷����������(
)�׳ơ����������������������谷����������( )�����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
)�����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
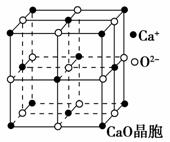
(4)CaO������ͼ��ʾ��CaO������Ca2������λ��Ϊ________��CaO�����NaCl����ľ����ֱܷ�Ϊ��CaO 3 401 kJ·mol��1��NaCl 786 kJ·mol��1���������߾����ܲ������Ҫԭ����__________________________________________________________________________________________________________________________________________��
������(1)Ca���������2�����ӣ�����ͬһ���ڵ�Ԫ���ڵ������ڣ��ڲ�����������K��M��ֱ�Ϊ2��8��18�����ӣ������Ļ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s2��[Ar]3d104s2����CN ��Ϊ�ȵ����壬�������ԭ������ȣ��۵���������ȣ��ʿ�����CO2����ΪCO2��ֱ���η��ӣ���CN
��Ϊ�ȵ����壬�������ԭ������ȣ��۵���������ȣ��ʿ�����CO2����ΪCO2��ֱ���η��ӣ���CN �Ŀռ乹����ֱ���Ρ�(2)���ط��ӵĽṹ��ʽΪ
�Ŀռ乹����ֱ���Ρ�(2)���ط��ӵĽṹ��ʽΪ �������е�Cԭ�Ӳ���sp2�ӻ���ʽ��̼��֮��Ĺ��ۼ���1���Ҽ���1���м���(3)���������������谷����֮��ͨ�����Ӽ�������϶��γɽ�ʯ��(4)�ɾ���ͼ�ɿ�����1��Ca2����Χ��6��O2����������Ca2������λ��Ϊ6��CaO����ľ�����Զ����NaCl����ľ����ܵ�ԭ����CaO������Ca2����O2���Ĵ���������NaCl������Na����Cl���Ĵ�������
�������е�Cԭ�Ӳ���sp2�ӻ���ʽ��̼��֮��Ĺ��ۼ���1���Ҽ���1���м���(3)���������������谷����֮��ͨ�����Ӽ�������϶��γɽ�ʯ��(4)�ɾ���ͼ�ɿ�����1��Ca2����Χ��6��O2����������Ca2������λ��Ϊ6��CaO����ľ�����Զ����NaCl����ľ����ܵ�ԭ����CaO������Ca2����O2���Ĵ���������NaCl������Na����Cl���Ĵ�������
�𰸡�(1)1s22s22p63s23p63d104s2��[Ar]3d104s2��CO2��ֱ���Ρ�(2)sp2�� ��(3)���Ӽ����
��(3)���Ӽ����
(4)6��CaO������Ca2����O2���Ĵ���������NaCl������Na����Cl���Ĵ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ��һ���ܴ����������(����)
A��ʹ���ȱ��ɫ����Һ��Mg2����K����SO ��NO
��NO
B��ʹ��̪���ɫ����Һ��Na����Cu2����HCO ��NO
��NO
C��0.1 mol·L��1 AgNO3��Һ��H����K����SO ��I��
��I��
D��0.1 mol·L��1 NaAlO2��Һ��H����Na����Cl����SO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����(����)
A�����Ȼ�����Һ��ʴͭ�壺Cu��Fe3��===Cu2����Fe2��
B����ˮ��ͨ��������SO2��I2��SO2��2H2O===2I����SO ��4H��
��4H��
C����������Һ�еμӹ�����ˮ��Ag����NH3·H2O===AgOH����NH
D����KAl(SO4)2��Һ�е���Ba(OH)2��Һ�����������ʵ������Al3����2SO ��2Ba2����4OH��===AlO
��2Ba2����4OH��===AlO ��2BaSO4����2H2O
��2BaSO4����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
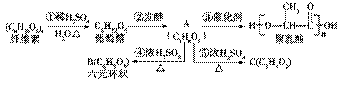
�������ǹ������˫�����Ը߷��ӣ�������ת����ϵ��
�Իش��������⡣
(1)A�й����ŵ�����Ϊ________________��
(2)д��A��2��ͬ���칹��Ľṹ��ʽ��________��
(3)�ٵķ�Ӧ������________���ݵķ�Ӧ������________��
(4)д���йط�Ӧ�Ļ�ѧ����ʽ��
��____________����____________��
(5)�����һ�����÷����ľ��������ϵ����룺 _____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������йص���(����)
��NH3���ۡ��е�Ȣ�A������Ԫ���⻯��ĸߡ���С���ӵĴ���������Ժ�ˮ������Ȼ��ܡ��۱����ܶȱ�Һ̬ˮ���ܶ�С�������ص��ۡ��е�ȴ���ĸߡ������ǻ���������ۡ��е�ȶ��ǻ�������ĵ͡���ˮ���Ӹ����º��ȶ�
A���٢ڢۢܢݢ� B���٢ڢۢܢ�
C���٢ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������е����Ų�ʽ��ԭ���У��뾶������(����)
A��1s22s22p63s23p1 B��1s22s22p5
C��1s22s22p63s23p4 D��1s22s22p1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ������Ϊ83��Ԫ��λ�ڣ��ٵ�5���ڣ��ڵ�6���ڣ��ۢ�A�壻�ܢ�A�壻�ݢ�B�壬������ȷ�������(����)
A���٢� B���ڢ� C���ڢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���ȷ�Ӧ����Ҫ���Ⱦ��ܷ�Ӧ�����ȷ�Ӧ�����ȾͲ��ܷ�Ӧ(����)
(2)���ʷ�����ѧ�仯�����������ı仯(����)
(3)���������仯�����ʱ仯���ǻ�ѧ�仯(����)
(4)���ȷ�Ӧ���κ����������ܷ���(����)
(5)Naת��ΪNa��ʱ�����յ��������Ǹù��̵ķ�Ӧ��(����)
(6)ˮ������ΪҺ̬ˮʱ�ų����������Ǹñ仯�ķ�Ӧ��(����)
(7)ͬ��ͬѹ�£���ӦH2(g)��Cl2(g)===2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ(����)
(8)���淴Ӧ�Ħ�H��ʾ��ȫ��Ӧʱ�������仯���뷴Ӧ�Ƿ������(����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com