

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”³¤É³ŹŠøßČżµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠø÷×éĄė×ÓŌŚÖø¶ØČÜŅŗÖŠ£¬æÉÄÜ“óĮæ¹²“ęµÄŹĒ£Ø £©
¢ŁČÜÓŠ×ćĮæ°±ĘųµÄĪŽÉ«ČÜŅŗÖŠ£ŗK+£¬NH £¬Na+£¬HSO
£¬Na+£¬HSO £¬PO
£¬PO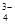 £¬MnO
£¬MnO
¢ŚÄÜŹ¹pHŹŌÖ½±äÉīĄ¶É«µÄČÜŅŗÖŠ£ŗK+£¬Na+£¬AlO £¬NO
£¬NO £¬S
£¬S £¬SO
£¬SO
¢ŪĖ®µēĄėµÄH+ÅضČc(H+)=10-13mol”¤L-1µÄČÜŅŗÖŠ£ŗCl £¬CO
£¬CO £¬S2O
£¬S2O £¬Fe3+£¬K+£¬Na+
£¬Fe3+£¬K+£¬Na+
¢Ü¼ÓČėAlÄܷųöH2µÄČÜŅŗÖŠ£ŗNa+£¬NH £¬K+£¬SO
£¬K+£¬SO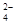 £¬Cl
£¬Cl £¬Br
£¬Br
¢ŻŹ¹ŹÆČļ±äŗģµÄČÜŅŗÖŠ£ŗFe3+£¬Na+£¬K+£¬NO £¬SO
£¬SO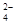 £¬Cl
£¬Cl
¢ŽŹŅĪĀĻĀĻĀpH=1µÄČÜŅŗÖŠ£ŗFe2+£¬Al3+£¬K+£¬Na+£¬NO £¬I
£¬I £¬Cl
£¬Cl £¬S
£¬S
¢ßŗ¬ÓŠ“óĮæFe3+µÄČÜŅŗ£ŗK+£¬NH £¬Na+£¬SCN
£¬Na+£¬SCN £¬I
£¬I £¬Br
£¬Br
¢ąŹŅĪĀĻĀ£¬ =0.1 mol/LµÄČÜŅŗÖŠ£ŗNa+£¬K+£¬SO
=0.1 mol/LµÄČÜŅŗÖŠ£ŗNa+£¬K+£¬SO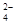 £¬SiO
£¬SiO £¬NO
£¬NO
A£®¢Ł¢Ū¢Ü¢Ž B£®¢Ł¢Ū¢Ž¢ß C£®¢Ś¢Ü¢Ż¢ą D£®¢Ś¢Ż¢ß¢ą
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com