



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
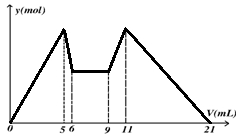
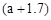 g”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
g”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒA£®Ć¾ĀĮŗĻ½šÓėŃĪĖį·“Ó¦×ŖŅʵē×Ó×ÜŹżĪŖ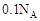 |
B£®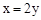 |
C£®³ĮµķŹĒ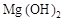 ŗĶ ŗĶ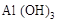 µÄ»ģŗĻĪļ µÄ»ģŗĻĪļ |
| D£®1.2£¾a£¾0.9 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŹµŃé²½Öč | ŹµŃéĻÖĻó |
| ¢Ł½«“ņÄ„¹żµÄĀĮʬ£Ø¹żĮ棩·ÅČėŅ»¶ØÅØ¶ČµÄCuCl2ČÜŅŗÖŠ”£ | ²śÉśĘųÅŻ£¬Īö³öŹčĖɵÄŗģÉ«¹ĢĢ壬ČÜŅŗÖš½„±äĪŖĪŽÉ«”£ |
| ¢Ś·“Ó¦½įŹųŗó·ÖĄė³öČÜŅŗ±øÓĆ”£ | |
| ¢ŪŗģÉ«¹ĢĢåÓĆÕōĮóĖ®Ļ“µÓŗó£¬ÖĆÓŚ³±ŹŖæÕĘųÖŠ”£ | Ņ»¶ĪŹ±¼äŗó¹ĢĢåÓÉŗģÉ«±äĪŖĀĢÉ«[ŹÓĘäÖ÷ŅŖ³É·ÖĪŖCu2(OH)2CO3]”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
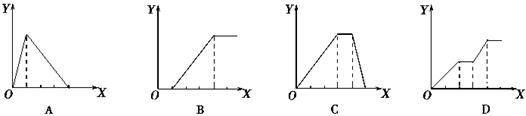
| A£®ĻņAlCl3ČÜŅŗÖŠÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮæĒŅ±ßµĪ±ßÕńµ“ |
| B£®ĻņNaAlO2ČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖįÖĮ¹żĮæĒŅ±ßµĪ±ßÕńµ“ |
| C£®ĻņNH4Al(SO4)2ČÜŅŗÖŠÖšµĪ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗÖ±ÖĮ¹żĮæ |
| D£®ĻņNaOH”¢Ba(OH)2”¢NaA1O2µÄ»ģŗĻČÜŅŗÖŠÖš½„ĶØČė¶žŃõ»ÆĢ¼ÖĮ¹żĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| | ¼×Ķ¬Ń§ | ŅŅĶ¬Ń§ |
| Ń”ÓĆŹŌ¼Į | A1C13ČÜŅŗ”¢NaOHČÜŅŗ | A12(SO4)3ČÜŅŗ”¢°±Ė® |
| ²Ł×÷ | ĻņAlCl3ČÜŅŗÖŠÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮæ | ĻņA12(SO4)3ČÜŅŗÖŠÖšµĪ¼ÓČė°±Ė®ÖĮ¹żĮæ |
| ŹµŃéĻÖĻó | | |
| »Æѧ·½³ĢŹ½ | | A12(SO4)3+6NH3”¤H2O=2A1(OH)3 ”ż+3(NH4)2SO4 |
| Ąė×Ó·½³ĢŹ½ | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
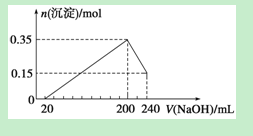
| A£®ÉĻŹöÓÉMgŗĶAl×é³ÉµÄ»ģŗĻĪļµÄÖŹĮæĪŖ8g |
| B£®ĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ1 mol”¤L£1 |
| C£®Éś³ÉµÄH2ŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ11.2L |
| D£®NaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ3.75 mol”¤L£1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
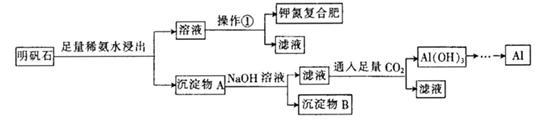
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¶µ×ÅŅŗĢåµÄÕā²ćĤŹĒŃõ»ÆĀĮ |
| B£®øĆŹµŃéĖµĆ÷ĮĖŃõ»ÆĀĮµÄČŪµćøßÓŚĀĮµÄČŪµć |
| C£®ĀĮ¼«Ņ×ÓėæÕĘųÖŠµÄŃõĘų·“Ó¦ŌŚ±ķĆęŠĪ³ÉŃõ»ÆĪļ±£»¤Ä¤ |
| D£®ĀĮµÄ»ÆѧŠŌÖŹ½ĻĪČ¶Ø£¬¼“Ź¹¼ÓČȵ½ČŪ»ÆŅ²²»ÓėæÕĘųÖŠµÄŃõĘų·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com