
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
| 582.4L |
| 22.4L/mol |


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һʱ����ȡ�ζ���ĩ����ʱ�����ӿ̶��� |
| B���ⶨ����ͭ�����нᾧˮ������ʵ��ʱ������ʱ�����δ��ȫ��� |
| C���к͵ζ�ʱ���Ӵ���Һǰ��ƿ��������ˮ |
| D���ⶨ1mol��������IJ����У���Ӧ������δ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 4 |
| �ܽ� |
| �� |
| BaCl2 |
| �� |
| NaOH |
| �� |
| Na2CO3 |
| �� |
| ���� |
| �� |
| ����ʳ�� |
| �� |
| �������ᾧ����� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
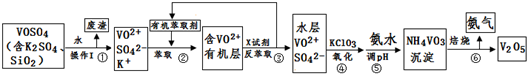
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
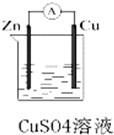 ij��ѧ��ȤС��������ͼ��ʾԭ���װ�ý���ʵ�飬��ش��������⣺
ij��ѧ��ȤС��������ͼ��ʾԭ���װ�ý���ʵ�飬��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
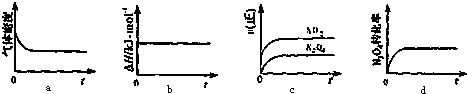
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
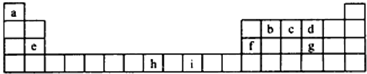
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com