

���� ��1��������Һ�еĻ�ѧ��Ӧ�������¶Ȼ���������������Ӧ���ʣ�
��2�����ݷ�Ӧ���̿�֪����Ӧ1Ϊ�����������������±���������ԭ��ClO2������Ԫ���غ�͵���غ�д���ӷ���ʽ��
��3����Ӧ2Ϊ��ClO2�ڼ��������±�˫��ˮ��ԭ��NaClO2������ClԪ�ػ��ϼ۵ı仯�ж�������������Ԫ���غ�д��ѧ����ʽ��
��4����ѹ�����ڽϵ��¶��¿ɽ��У���ֹ�¶ȹ��߶��������ʷֽ⣻
��5������Ϊ����Na2S2O3��Һ�ζ����յ㣬��Ӧ��I2+2S2O32-=2I-+S4O62-��I2��ָʾ�����۲�����ɫ�����յ��ǵμ����һ��Һ��ʱ��Һ����ɫ�����ɫ�Ұ�����ڲ���ɫ��
���ɷ�Ӧ�е�IԪ���غ��֪��ClO2-��2I2��4 S2O32-��25.00mL������Һ��n��NaClO2��=$\frac{1}{4}$��cV��10-3mol��m��NaClO2��=$\frac{1}{4}$��90.5cV��10-3g����Ʒmg���250mL������Һ�е�NaClO2��������10��������õ�NaClO2������������
��� �⣺��1�����������Һ��ķ�Ӧ��Ϊ��߷�Ӧ���ʣ����ʵ����߷�Ӧ�¶ȣ���������ҺŨ�ȣ�����SO2������Һ�ĽӴ������
�ʴ�Ϊ���ʵ����߷�Ӧ�¶ȣ���������ҺŨ�ȵȣ�
��2�����ݷ�Ӧ���̿�֪����Ӧ1Ϊ�����������������±���������ԭ��ClO2����Ӧ�����ӷ���ʽΪ2ClO3-+SO2�T2ClO2+SO42-��
�ʴ�Ϊ��2ClO3-+SO2�T2ClO2+SO42-��
��3����Ӧ2ΪClO2�ڼ��������±�˫��ˮ��ԭ��NaClO2����Ӧ�ķ���ʽΪH2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2����Ӧ��ClԪ�صĻ��ϼ۽��ͣ���ClO2Ϊ��������
�ʴ�Ϊ��ClO2��H2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2��
��4����ѹ�����ڽϵ��¶��¿ɽ��У���ֹ��ѹ�����¶ȹ��ߣ������������ֽ⣬�ʴ�Ϊ����ѹ�����¶ȹ��ߣ������������ֽ⣻
��5������Ϊ����Na2S2O3��Һ�ζ����յ㣬��Ӧ��I2+2S2O32-=2I-+S4O62-��I2��ָʾ�����۲�����ɫ�����յ��ǵμ����һ��Һ��ʱ��Һ����ɫ�����ɫ�Ұ�����ڲ���ɫ��
�ʴ�Ϊ���μ����һ��Һ��ʱ��Һ����ɫ�����ɫ�Ұ�����ڲ���ɫ��
���ɷ�Ӧ�е�IԪ���غ��֪��ClO2-��2I2��4 S2O32-��25.00mL������Һ��n��NaClO2��=$\frac{1}{4}$��cV��10-3mol��m��NaClO2��=$\frac{1}{4}$��90.5cV��10-3g����Ʒmg���250mL������Һ�е�NaClO2��������10��������Ʒ��NaClO2����������Ϊ$\frac{\frac{1}{4}��90.5cV��1{0}^{-2}g}{mg}$��100%=$\frac{22.625cV}{m}$%��
�ʴ�Ϊ��$\frac{22.625cV}{m}$%��
���� ���⿼�����ʵ��Ʊ�ʵ��Ĺ�ҵ��ƣ���Ŀ�Ѷ��еȣ�����ע��������ʵ����ʣ��������غ�ĽǶ��Լ�������ԭ��Ӧ���ص��ж������Ϊ������Ĺؼ���Ҳ���״��㣮


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | x=y+0.2 | B�� | x=$\frac{y}{2}$ | C�� | x=0.1+$\frac{y}{2}$ | D�� | x=y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
 ��С����Ƶ��Ʊ�װ����ͼ���г���������ȥ�����ش��������⣺
��С����Ƶ��Ʊ�װ����ͼ���г���������ȥ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
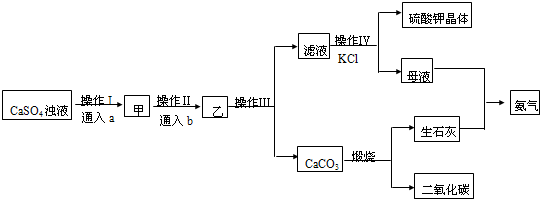
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2SO3��Һ��Ba��OH��2��Һ�� | B�� | FeCl2��Һ��KSCN��Һ�� | ||
| C�� | KI��������Һ�� | D�� | NaOH��Һ��Ba��OH��2��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�㶼������?���� | B�� | ���н������� | ||
| C�� | �����нϸߵ��۵� | D�� | һ�㶼����չ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com