
| A����ʹ���Ը��������Һ��ɫ | B����ʹ���CCl4��Һ��ɫ |
| C��һ�������£��ܹ�������ȥ��Ӧ | D��һ�������£��ܹ�����ȡ����Ӧ |
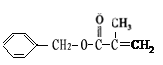
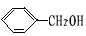 + O2
+ O2 2 C6H5CHO��2H2O;
2 C6H5CHO��2H2O; CH3CH(CH3)COOCH2CH3+ H2O
CH3CH(CH3)COOCH2CH3+ H2O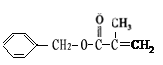 ��
�� + O2
+ O2 2 C6H5CHO��2H2O��
2 C6H5CHO��2H2O�� CH3CH(CH3)COOCH2CH3+ H2O��
CH3CH(CH3)COOCH2CH3+ H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
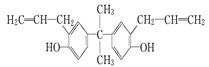
| A�����л������ڷ����廯�������ʽΪC21H24O2 |
| B�����л������������̼ԭ���п�����ͬһƽ���� |
| C��˫��A����ʹ��ˮ��ɫ������ʹ���Եĸ��������Һ��ɫ |
| D��˫��A��һ��ȡ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
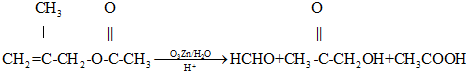
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
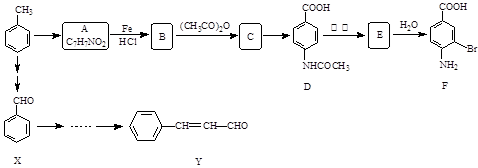
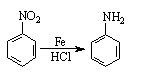 ������
������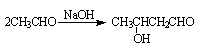 ��
��| A������ʽ��C7H7NO2Br | B�����������ᷴӦ������NaOH��Һ��Ӧ |
| C���ܷ���������Ӧ | D��1 mol F����������2 mol NaOH |
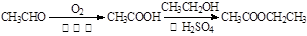
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
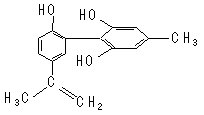
| A����FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ�� |
| B������KMnO4(H+)��Һ���۲���ɫ��ȥ����֤���ṹ�д���̼̼˫�� |
| C���÷����е�����ԭ���п��ܹ�ƽ�� |
| D��1 mol��������Ũ��ˮ��H2��Ӧ�������Br2��H2�ֱ�Ϊ4 mol��7 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ϩˮ�����Ҵ���������ˮ�����Ҵ� |
| B���ױ�ʹ���Ը��������Һ��ɫ����Ȳʹ������Ȼ�̼��Һ��ɫ |
| C���Ҵ���ˮ����ϩ����������NaOH�Ҵ���Һ��������ϩ |
| D����������������������������������ȡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
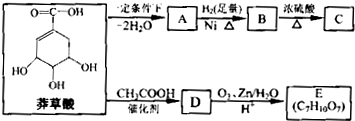
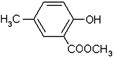
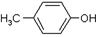 )���л���ҵ����Ҫԭ�ϣ������ںϳɶ����л��
)���л���ҵ����Ҫԭ�ϣ������ںϳɶ����л��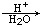 R��COOH��
R��COOH��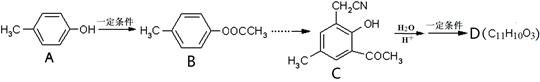
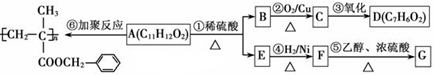
 ���ϳ�·������ͼΪ��
���ϳ�·������ͼΪ��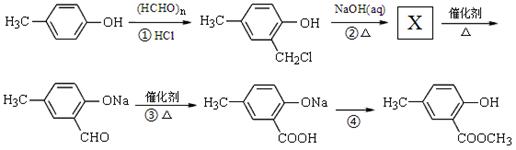
 �ĺϳ�·������ͼ��ʾ(�л����ýṹ��ʽ��ע����Ӧ�Լ�������)
�ĺϳ�·������ͼ��ʾ(�л����ýṹ��ʽ��ע����Ӧ�Լ�������)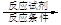 B����
B����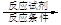 Ŀ�����)
Ŀ�����)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C4H10��C20H42 | B���ڶ��ױ��ͶԶ��ױ� |
| C��C4H8��C3H6 | D��һ�������1,2���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
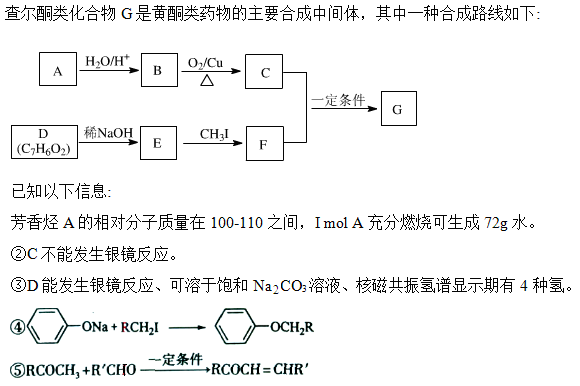
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com