
| A������Һ��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������϶��� |
| B������Һ��0.1 mol?L-1��CH3COOH��Һ������ʵ���Ũ�ȵ������NaOH��Һ��϶��� |
| C����������Һ�м���������NaOH��HCl��Һʱ����Һ��pH���ᷢ�������仯 |
| D����������Һ�м�������NaOH��Һ����ʹ��Һ������Ũ�ȸı�Ϊ��c��CH3COO-����c��Na+����c��OH-����c��H+�� |


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+��Na+��Cu2+��Al3+ |
| ������ | SO4 2-��HCO3-��NO3-OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����pH=5��H2SO4��Һϡ��1��103��c��H+����c��SO42-��=2��1 |
| B������������Ƶ�PH=7�Ļ����Һ�У�c��CH3COO-��+c��CH3COOH����c��Na+�� |
| C��0.1mol?L-1���������Һ�У�c��NH4+��+c��H+��=c��SO42-��+c��OH-�� |
| D��������pH=4��NaHC2O4��Һ�У�c��H2C2O4����c��C2O42-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ��ͨ��������Cl2+H2O?H++Cl-+HClO | ||||
B����������Һ�мӹ�����ˮ��Al3++4NH3?H2O=AlO
| ||||
| C������������Һ����μ�������������Һ������Һǡ�ó����ԣ�Ba2++OH-+SO42-+H+=BaSO4��+H2O | ||||
D����������6mol/L��������ˮ��Һ���ȣ�C2H5Br+OH-
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
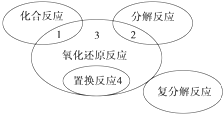 ���л�ѧ��Ӧ��������3���ǣ�������
���л�ѧ��Ӧ��������3���ǣ�������| A��4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | ||||
B��2NaHCO3
| ||||
C��4NH3+5O2
| ||||
D��Zn+H2SO4
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ҩ�ǰѲݵijɷ�֮һM��������ɱ�����ã�M�Ľṹ��ͼ��ʾ����������ȷ���ǣ�������
��ҩ�ǰѲݵijɷ�֮һM��������ɱ�����ã�M�Ľṹ��ͼ��ʾ����������ȷ���ǣ�������| A��M����Է���������178 |
| B��1mol M�������2mol Br2������Ӧ |
| C��M��������NaOH��Һ������Ӧʱ�������л�����Ļ�ѧʽΪC9H4O5Na4 |
| D��1mol M������NaHCO3��Ӧ������2mol CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��100��ʱ��pH=10��NaOH��Һ��pH=2��H2SO4ǡ���кͣ�������Һ��pH=7 |
| B��25��ʱ��0.2mol?L-1Ba��OH��2��Һ��0.2mol?L-1HCl�������ϣ�������Һ��pH=7 |
| C��25��ʱ��0.2mol?L-1NaOH��Һ��0.2mol?L-1CH3COOHǡ���кͣ�������Һ��pH=7 |
| D��25��ʱ��pH=12�İ�ˮ��pH=2��H2SO4�������ϣ�������Һ��pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2mol | B��3mol |
| C��4mol | D��6mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������ͬ��Na2S2O3��Һ��H2SO4��Һ��Ӧ��������Һ���¶ȣ��������������ʱ�����̣���������������ʱ�����߷�Ӧ�¶ȣ���ѧ��Ӧ���ʼӿ� |
| B����ҵ�������У���SO3�����սΣ���������Ҫװ��ɻ���������Һ�Ӵ������ʹSO3�������������� |
| C�����ݻ��ɱ���ܱ������з�����Ӧ��2NH3��g��?N2H4��l��+H2��g�������ݻ��������Сһ�룬����Ӧ���ʼӿ죬�淴Ӧ���ʼ��� |
| D��A��B��֧�Թ��зֱ��������5%��H2O2��Һ����B�Թ��м���2��3��FeCl3��Һ��B�Թ��в������ݿ죬��������������ʱ���������Ըı仯ѧ��Ӧ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com