
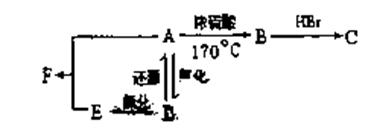
 ��2��������C�Ľṹ��ʽΪ
��2��������C�Ľṹ��ʽΪ 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
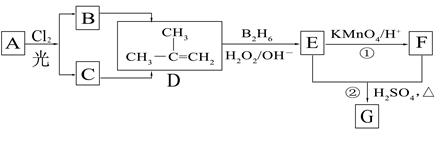
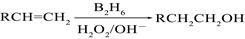 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
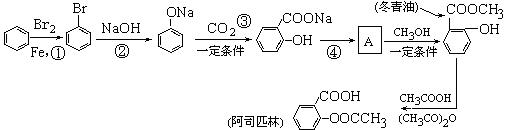
 ��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ��
��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ�� ___________________________________________��
___________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
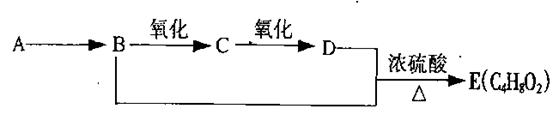
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��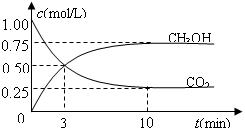
| A�������¶� | B������He(g)��ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1mol H2 |
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ������������������������������������������������������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ϩͨ�����Ը��������Һ | B�����Ҵ���ȡ��ϩ |
| C���Ҵ�����ͨ�����ȵ�����ͭ | D����ȩ�������ӳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��ͬѹ��4.75����������Իش�
��ͬѹ��4.75����������Իش� ��Ľṹ��ʽΪ ��
��Ľṹ��ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��˵���У���ȷ����
��˵���У���ȷ����| A����������һ�ִ����� |
| B���䵥����CH3CH(OH)COOH |
| C��ͨ�������Ʊ�������ķ�Ӧ�ǼӾ۷�Ӧ[ |
| D���������Ʊ�������ԭ��������Ϊ100% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com