
ÉčnA“ś±ķ°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ””””
A£®1 molĮņĖį¼ŲÖŠŅõĄė×ÓĖł“ųµēŗÉŹżĪŖnA
B£®ŅŅĻ©ŗĶ»·±ūĶéC3H6×é³ÉµÄ28 g»ģŗĻĘųĢåÖŠŗ¬ÓŠ3nAøöĒāŌ×Ó
C£®±ź×¼×“æöĻĀ£¬22.4 LĀČĘųÓė×ćĮæĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦×ŖŅʵĵē×ÓŹżĪŖnA
D£®½«0.1 molĀČ»ÆĢśČÜÓŚ1 LĖ®ÖŠ£¬ĖłµĆČÜŅŗŗ¬ÓŠ0.1nAøöFe3£«

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«ŗ£Ė®µ»ÆÓėÅØŗ£Ė®×ŹŌ“»Æ½įŗĻĘšĄ“ŹĒ×ŪŗĻĄūÓĆŗ£Ė®µÄÖŲŅŖĶ¾¾¶Ö®Ņ»”£Ņ»°ćŹĒĻČ½«ŗ£Ė®µ»Æ»ńµĆµĖ®£¬ŌŁ“ÓŹ£ÓąµÄÅØŗ£Ė®ÖŠĶعżŅ»ĻµĮŠ¹¤ŅÕĢįČ”ĘäĖū²śĘ·”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĻĀĮŠøĽųŗĶÓÅ»Æŗ£Ė®×ŪŗĻĄūÓĆ¹¤ŅÕµÄÉčĻėŗĶ×ö·ØæÉŠŠµÄŹĒ £ØĢīŠņŗÅ£©”£
¢ŁÓĆ»ģÄż·Ø»ńČ”µĖ® ¢ŚĢįøß²æ·Ö²śĘ·µÄÖŹĮæ
¢ŪÓÅ»ÆĢįČ”²śĘ·µÄĘ·ÖÖ ¢ÜøĽų¼Ų”¢äå”¢Ć¾µÄĢįČ”¹¤ŅÕ
£Ø2£©²ÉÓĆ”°æÕĘų“µ³ö·Ø”±“ÓÅØŗ£Ė®ÖŠ“µ³öBr2£¬²¢ÓĆ“æ¼īĪüŹÕ”£¼īĪüŹÕäåµÄÖ÷ŅŖ·“Ó¦ŹĒBr2+Na2CO3+H2O 
 NaBr + N
NaBr + N aBrO3+NaHCO3£¬ĪüŹÕ1mol Br2Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ mol”£
aBrO3+NaHCO3£¬ĪüŹÕ1mol Br2Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ mol”£
£Ø3£©ŗ£Ė®ĢįĆ¾µÄŅ»¶Ī¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ
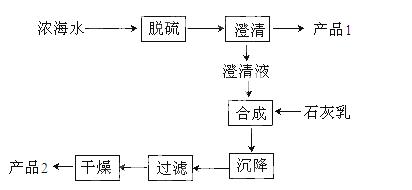
ÅØŗ£Ė®µÄÖ÷ŅŖ³É·ÖČēĻĀ£ŗ
| Ąė×Ó | N | Mg2+ | Cl- | SO42- |
| ÅضČ/g/L | 63.7 | 28.8 | 144.6 | 46.4 |
øĆ¹¤ŅÕ¹ż³ĢÖŠ£¬ĶŃĮņ½×¶ĪÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬²śĘ·2µÄ»ÆѧŹ½ĪŖ £¬1LÅØŗ£Ė®×ī¶ąæɵƵ½²ś Ę·2µÄÖŹĮæĪŖ g”£
Ę·2µÄÖŹĮæĪŖ g”£
£Ø4£©²ÉÓĆŹÆÄ«Ńō¼«”¢²»ŠāøÖŅõ¼«µē½āČŪČŚµÄĀČ»ÆĆ¾£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»µē½āŹ±£¬ČōÓŠÉŁĮæĖ®“ęŌŚ»įŌģ³É²śĘ·Ć¾µÄĻūŗÄ£¬Š“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ō×ÓŠņŹżŅĄ“ĪŌö“óµÄĖÄÖÖŌŖĖŲA”¢B”¢C”¢D·Ö±š“¦ÓŚµŚŅ»ÖĮµŚĖÄÖÜĘŚ£¬×ŌČ»½ēÖŠ“ęŌŚ¶ąÖÖAµÄ»ÆŗĻĪļ£¬BŌ×ÓŗĖĶāµē×ÓÓŠ6ÖÖ²»Ķ¬µÄŌĖ¶ÆדĢ¬£¬BÓėCæÉŠĪ³ÉÕżĖÄĆęĢåŠĪ·Ö×Ó£¬DµÄ»łĢ¬Ō×ÓµÄ×īĶāÄܲćÖ»ÓŠŅ»øöµē×Ó£¬ĘäĖūÄÜ²ć¾łŅŃ³äĀśµē×Ó”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÕāĖÄÖÖŌŖĖŲÖŠµēøŗŠŌ×ī“óµÄŌŖĖŲ£¬Ę仳Ģ¬Ō×ӵļŪµē×ÓÅŲ¼Ķ¼ĪŖ________£¬µŚŅ»µēĄėÄÜ×īŠ”µÄŌŖĖŲŹĒ________(ĢīŌŖĖŲ·ūŗÅ)”£
(2)CĖłŌŚÖ÷×åµÄĒ°ĖÄÖÖŌŖĖŲ·Ö±šÓėAŠĪ³ÉµÄ»ÆŗĻĪļ£¬·ŠµćÓÉøßµ½µĶµÄĖ³ŠņŹĒ________(Ģī»ÆѧŹ½)£¬³ŹĻÖČē“ĖµŻ±ä¹ęĀɵÄŌŅņŹĒ___________________”£
(3)BŌŖĖŲæÉŠĪ³É¶ąÖÖµ„ÖŹ£¬Ņ»ÖÖ¾§Ģå½į¹¹ČēĶ¼Ņ»ĖłŹ¾£¬ĘäŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ________”£ĮķŅ»Öֵľ§°ūČēĶ¼¶žĖłŹ¾£¬Čō“Ė¾§°ūÖŠµÄĄā³¤ĪŖ356.6 pm£¬Ōņ“Ė¾§°ūµÄĆܶČĪŖ________________________________________________g·c m£3(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£(
m£3(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£(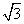 £½1.732)
£½1.732)
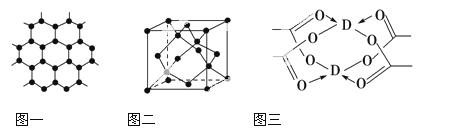
(4)DŌŖĖŲŠĪ³ÉµÄµ„ÖŹ£¬Ę侧ĢåµÄ¶Ń»żÄ£ŠĶĪŖ________£¬DµÄ“×ĖįŃĪ¾§Ģå¾Ö²æ½į¹¹ČēĶ¼Čż£¬øĆ¾§ĢåÖŠŗ¬ÓŠµÄ»Æѧ¼üŹĒ________(ĢīŃ”ĻīŠņŗÅ)”£
¢Ł¼«ŠŌ¼ü””””¢Ś·Ē¼«ŠŌ¼ü””””¢ŪÅäĪ»¼ü””””¢Ü½šŹō¼ü
(5)ĻņDµÄĮņĖįŃĪČÜŅŗÖŠµĪ¼Ó¹żĮæ°±Ė®£¬¹Ū²ģµ½µÄĻÖĻóŹĒ________”£ĒėŠ“³öÉĻŹö¹ż³ĢµÄĄė×Ó·½³ĢŹ½£ŗ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČżøöĆܱÕČŻĘ÷ÖŠ·Ö±š³äČėNe”¢H2”¢O2ČżÖÖĘųĢ壬µ±ĖüĆĒµÄĪĀ¶ČŗĶĆܶȶ¼ĻąĶ¬Ź±£¬ÕāČżÖÖĘųĢåµÄŃ¹Ēæ“ӓ󵽊”µÄĖ³ŠņŹĒ
A£®p(Ne) £¾p(H2) £¾p(O2) B£®p(O2) £¾p(Ne) £¾p(H2)
C£®p(H2) £¾p(O2) £¾p(Ne) D£®p(H2) £¾p(Ne) £¾p(O2)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¶ŌÓŠ¹ŲÖŖŹ¶µÄĄķ½āÕżČ·µÄŹĒ(””””)
A£®ĪļÖŹµÄĮæŹĒĪļÖŹĖłŗ¬Ī¢Į£µÄŹżĮæ
B£®1Ħ¶ūŃõĘųµÄÖŹĮæµČÓŚNAøöO2·Ö×ÓµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®ŗĶ
C£®°¢·ü¼ÓµĀĀŽ³£ŹżŹĒČĖĆĒ¹ę¶ØµÄŹż£¬Ć»ÓŠµ„Ī»
D£®µ±H2µÄĦ¶ūÖŹĮæŅŌg·mol£1ĪŖµ„Ī»Ź±£¬ŌŚŹżÖµÉĻÓėH2µÄĻą¶Ō·Ö ×ÓÖŹĮæĻąµČ
×ÓÖŹĮæĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ20”ꏱŅ»øÖŠŌČŻĘ÷ÄŚ²æÓŠŅ»øö²»Ā©ĘųĒŅæÉ»¬¶ÆµÄ»īČū½«ČŻĘ÷·Öøō³É×óÓŅĮ½ŹŅ”£×óŹŅ³äČėN2£¬ÓŅŹŅ³äČėH2ŗĶO2£¬»īČūÕżŗĆĶ£ĮōŌŚĄė×ó¶ĖµÄ1/4“¦£ØČēĶ¼¼×ĖłŹ¾£©£¬Č»ŗóµćČ¼H2ŗĶO2£¬·“Ó¦Ķź±Ļ ŗó»Öø“µ½ŌĄ“ĪĀ¶Č£¬»īČūĒ”ŗĆĶ£ŌŚÖŠ¼ä(ČēĶ¼ŅŅĖłŹ¾)£¬Ė®ÕōĘųµÄĢå»żæÉŗöĀŌ£¬Ōņ·“Ó¦Ē°H2ŗĶO2µÄĢå»ż±ČæÉÄÜŹĒ£Ø £©
ŗó»Öø“µ½ŌĄ“ĪĀ¶Č£¬»īČūĒ”ŗĆĶ£ŌŚÖŠ¼ä(ČēĶ¼ŅŅĖłŹ¾)£¬Ė®ÕōĘųµÄĢå»żæÉŗöĀŌ£¬Ōņ·“Ó¦Ē°H2ŗĶO2µÄĢå»ż±ČæÉÄÜŹĒ£Ø £©


Ķ¼¼× Ķ¼ŅŅ
¢Ł3”Ć4 ¢Ś4”Ć5 ¢Ū6”Ć2 ¢Ü7”Ć2
A£®¢Ł¢Ū B£®¢Ś¢Ü C£®¢Ł¢Ś D£®¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1.28 gijĘųĢåŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖ1.204”Į1 022£¬ŌņøĆĘųĢåµÄĦ¶ūÖŹĮæĪŖ(””””)
022£¬ŌņøĆĘųĢåµÄĦ¶ūÖŹĮæĪŖ(””””)
A£®64 g B. 64
C£®64 g/mol D£®32 g/mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®lmol FeI2Óė×ćĮæĀČĘų·“Ó¦Ź±×ŖŅʵĵē×ÓŹżĪŖ2NA
B£®2 L0.5 mol • L£1ĮņĖį¼ŲČÜŅŗÖŠŅõĄė×ÓĖł“ųµēŗÉŹżĪŖNA
C£®1 mol Na2O2¹ĢĢåÖŠŗ¬Ąė×Ó×ÜŹżĪŖ4NA
D£®±ūĻ©ŗĶ»·±ūĶé×é³ÉµÄ42 g»ģŗĻĘųĢåÖŠĒāŌ×ÓµÄøöŹżĪŖ6  NA
NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°ė ĶøĤÓėĀĖÖ½Ņ»ŃłÓŠŠķ¶ąŠ”æ×£¬µ«æ×¾¶±ČĀĖÖ½Š”£¬ĪŖÖ¤Źµ°ėĶøĤֻÄÜĶعżĄė×ÓŗĶ½ĻŠ”µÄ·Ö×Ó£¬½ŗĢå·ÖÉ¢ÖŹ²»ÄÜĶعż°ėĶøĤ”£ĻÖ°Ń10 mLµķ·Ū½ŗĢåŗĶ50 mL KClČÜŅŗµÄ»ģŗĻŅŗĢå¼ÓČėÓĆ°ėĶøĤÖĘ³ÉµÄ“üÄŚ£¬½«“Ė“ü½žČėÕōĮóĖ®ÖŠ”£2 minŗó£¬ÓĆĮ½Ö§ŹŌ¹Üø÷Č”5 mLÉÕ±ÖŠµÄŅŗĢå×öČēĻĀŹµŃ锣
ĶøĤÓėĀĖÖ½Ņ»ŃłÓŠŠķ¶ąŠ”æ×£¬µ«æ×¾¶±ČĀĖÖ½Š”£¬ĪŖÖ¤Źµ°ėĶøĤֻÄÜĶعżĄė×ÓŗĶ½ĻŠ”µÄ·Ö×Ó£¬½ŗĢå·ÖÉ¢ÖŹ²»ÄÜĶعż°ėĶøĤ”£ĻÖ°Ń10 mLµķ·Ū½ŗĢåŗĶ50 mL KClČÜŅŗµÄ»ģŗĻŅŗĢå¼ÓČėÓĆ°ėĶøĤÖĘ³ÉµÄ“üÄŚ£¬½«“Ė“ü½žČėÕōĮóĖ®ÖŠ”£2 minŗó£¬ÓĆĮ½Ö§ŹŌ¹Üø÷Č”5 mLÉÕ±ÖŠµÄŅŗĢå×öČēĻĀŹµŃ锣
£Ø1£©ĻņĘäÖŠŅ»Ö§ŹŌ¹ÜĄļµĪ¼ÓÉŁĮæAgNO3ČÜŅŗ£¬ĘäĻÖĻóŹĒ________________________________________________________________________”£
£Ø2£©ĻņĮķŅ»Ö§ŹŌ¹ÜĄļµĪ¼ÓÉŁĮæµāĖ®£¬ĘäĻÖĻóŹĒ________________________________________________________________________”£
£Ø3£©ÓÉÉĻŹöŹµŃéĻÖĻóµĆ³öµÄ½įĀŪŹĒ______________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com