
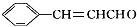 ���ڴ�������������1mol D��2mol H2��Ӧ���������ң����к�������-CH3
���ڴ�������������1mol D��2mol H2��Ӧ���������ң����к�������-CH3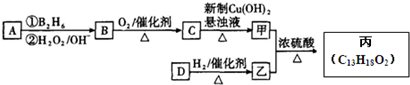
 ��
�� ��
������ �����Ϣ��ת����ϵ��֪AΪ�����������A����Է�������Ϊ56��������Cԭ����Ŀ�����=$\frac{56}{12}$=4��8����AΪC4H8�����ĺ˴Ź���������ʾֻ��2��壬����֪AΪ��CH3��2C=CH2 ��˳�ƿ�֪BΪCH3��2CHCH2OH��CΪ��CH3��2CHCHO����Ϊ��CH3��2CHCOOH�����ҷ�Ӧ�����л���C13H18O2��Ӧ�Ƿ���������Ӧ������Ϊ�������к���4��̼ԭ�ӣ��������к���9��̼ԭ�ӣ��ҵķ���ʽΪ��C9H12O��D���Է���������Ӧ������-CHO���ڴ�������������1mol D��2mol H2��Ӧ���������ң����к�������-CH3�������к���2������������û�м�������֪DΪ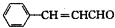 ����Ϊ
����Ϊ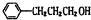 ���ݴ˽��
���ݴ˽��
��� �⣺�����Ϣ��ת����ϵ��֪AΪ�����������A����Է�������Ϊ56��������Cԭ����Ŀ�����=$\frac{56}{12}$=4��8����AΪC4H8�����ĺ˴Ź���������ʾֻ��2��壬����֪AΪ��CH3��2C=CH2 ��˳�ƿ�֪BΪCH3��2CHCH2OH��CΪ��CH3��2CHCHO����Ϊ��CH3��2CHCOOH�����ҷ�Ӧ�����л���C13H18O2��Ӧ�Ƿ���������Ӧ������Ϊ�������к���4��̼ԭ�ӣ��������к���9��̼ԭ�ӣ�F�ķ���ʽΪ��C9H12O��D���Է���������Ӧ������-CHO���ڴ�������������1mol D��2mol H2��Ӧ���������ң����к�������-CH3�������к���2������������û�м�������֪DΪ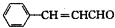 ����Ϊ
����Ϊ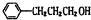 ��
��
��1��������������֪��A�Ľṹ��ʽΪ����CH3��2C=CH2 ��
�ʴ�Ϊ����CH3��2C=CH2 ��
��2��CΪ��CH3��2CHCHO��������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$��CH3��2CHCOONa+Cu2O��+3H2O��
�ʴ�Ϊ����CH3��2CHCHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$��CH3��2CHCOONa+Cu2O��+3H2O��
��3��DΪ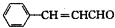 ��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹�壨�����������칹������������Ϊ-C��CHO��=CH2��Ҳ����Ϊ-CHO��-CH=CH2�����ڡ��䡢������λ�ù�ϵ���ʷ���������ͬ���칹�干��4�֣�
��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹�壨�����������칹������������Ϊ-C��CHO��=CH2��Ҳ����Ϊ-CHO��-CH=CH2�����ڡ��䡢������λ�ù�ϵ���ʷ���������ͬ���칹�干��4�֣�
�ʴ�Ϊ��4��
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ����
����
��5����Ϊ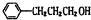 ����ͬ���칹�������������FeCl3��Һ����ɫ�����з��ǻ����䱽���ϵ�һ�����ֻ�����֣���ֻ����2���������������Ϊ-CH2CH2CH3��-CH��CH3��2���Ҵ��ڶ�λ���ʸ��л���Ľṹ��ʽΪ��
����ͬ���칹�������������FeCl3��Һ����ɫ�����з��ǻ����䱽���ϵ�һ�����ֻ�����֣���ֻ����2���������������Ϊ-CH2CH2CH3��-CH��CH3��2���Ҵ��ڶ�λ���ʸ��л���Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ���Ҫ�Ը������Ϣ�������ã��ܽϺõĿ���ѧ���Ķ���ȡ��Ϣ������ȷ��A�Ľṹ��ʽ�ǹؼ����������Ʒ������Ʒ����Ͻ����ƶϣ�ע�����չ����ŵ�������ת�����Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
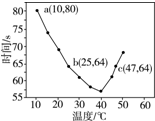
| A�� | 40��֮ǰ��40��֮����Һ������ʱ�����¶ȵı仯�����෴ | |
| B�� | ͼ��b��c�����Ӧ��NaHSO3�ķ�Ӧ������� | |
| C�� | ͼ��a���Ӧ��NaHSO3�ķ�Ӧ����Ϊ5.0��10-5 mol•��L•s��-1 | |
| D�� | �¶ȸ���40��ʱ�����۲���������ʵ���ָʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ƺͽ��ʯ | B�� | ���ɱ� | C�� | ������ˮ�� | D�� | ���ͱ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2=CH-CI | B�� | CH2=CH-CH=CH2 | C�� | CH3-CH=CH2 | D�� | 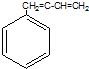 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��1����3����5����6�� | B�� | ��1����2����3����5�� | C�� | ��1����2����4����5�� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
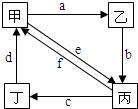 ������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| �� | �� | �� | �� | |
| �� | Cu | CuO | CuCl2 | Cu��NO3��2 |
| �� | Na2CO3 | NaOH | NaHCO3 | CO2 |
| �� | ��NH4��2SO3 | CaSO3 | SO2 | NH4HSO3 |
| �� | Al��OH��3 | Al2O3 | NaAlO2 | AlCl3 |
| A�� | �٢ڢۢ� | B�� | ���ڢۢ� | C�� | ���ڢ� | D�� | ���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѹ�����ڰ��ĺϳ� | |
| B�� | ʵ�����г����ű���ʳ��ˮ�ķ�ʽ�ռ����� | |
| C�� | ����ˮƿ�������ݴ���Һ��ð�� | |
| D�� | ��˫��ˮ�м�������������������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com