
 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��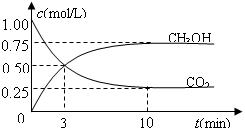
| A�������¶� | B������He(g)��ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1mol H2 |
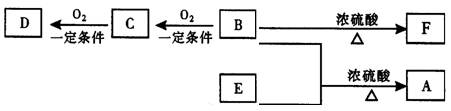
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ����������������������������������������������������������������� ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
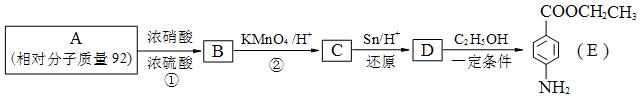
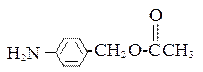 ��
��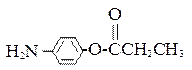 ��
�� �⣬�������������Ļ�����E��ͬ���칹���� �� �֡�
�⣬�������������Ļ�����E��ͬ���칹���� �� �֡� �ṹ�Ļ���
�ṹ�Ļ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
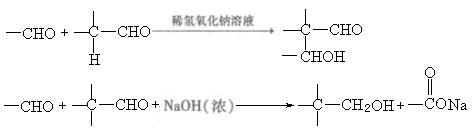
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��2��������C�Ľṹ��ʽΪ
��2��������C�Ľṹ��ʽΪ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 3RCOCl+H3PO3
3RCOCl+H3PO3 RCONH2+HCl
RCONH2+HCl| A����ʹ����KMnO4��Һ��ɫ�� | B���ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ���� |
| C��������������ụΪͬϵ� | D������H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ش�
�ش� ����Ľṹ��ʽ��_____________________��
����Ľṹ��ʽ��_____________________���鿴�𰸺ͽ���>>
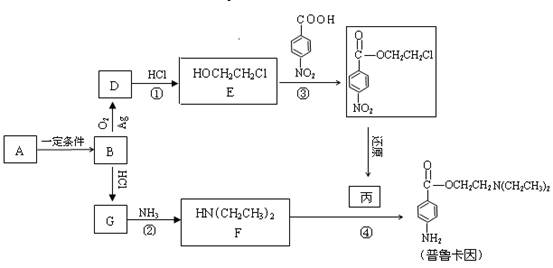
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

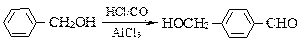 ��
��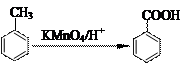
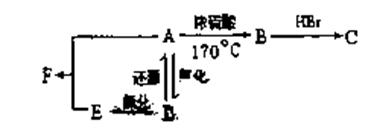
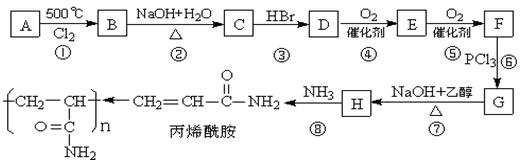
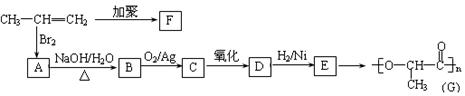
 ��������A~I��ת����ϵ����ͼ��
��������A~I��ת����ϵ����ͼ��
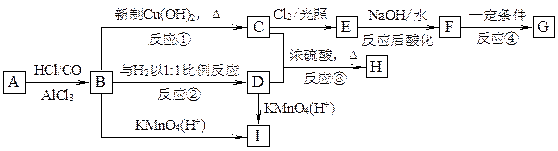
 ��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺
��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺ (1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� ��
(1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� �� (2)д���������ʵĽṹ��ʽ��F ��I ��A ��
(2)д���������ʵĽṹ��ʽ��F ��I ��A �� (3)д�����з�Ӧ�Ļ�ѧ����ʽ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ�� ��B��C�� ��
��B��C�� �� ��C+D��H�� ��
��C+D��H�� �� ��F��G�� ��
��F��G�� �� (4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��
(4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
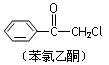



�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com