
| ŃōĄė×Ó | K£«”¢Na£«”¢Fe2£«”¢Ba2£«”¢NH4+”¢Ca2£« |
| ŅõĄė×Ó | OH£”¢NO3”Ŗ”¢I£”¢HCO3”Ŗ”¢AlO2”Ŗ”¢HSO4”Ŗ |
 BaSO4”ż£«NH3”ü£«2H2O
BaSO4”ż£«NH3”ü£«2H2O

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČėĀĮ·ŪÓŠĒāĘų²śÉśµÄČÜŅŗÖŠNa+”¢K+”¢SO42-”¢Cl-”¢HCO-3 |
| B£®ŗ¬ÓŠ“óĮæĻõĖįøłĄė×ÓµÄČÜŅŗÖŠH+”¢Fe2+”¢SO42-”¢Cl- |
| C£®³£ĪĀĻĀ£¬c(H+)/c(OH-) = 1”Į10-10µÄČÜŅŗÖŠNH4+”¢K+”¢Ca2+”¢Cl- |
| D£®³£ĪĀĻĀpH=1µÄČÜŅŗÖŠ£ŗMnO4-”¢NO3-”¢SO42-”¢Na+”¢Fe3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
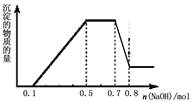
| A£®ČÜŅŗÖŠŅ»¶Ø²»ŗ¬CO32?£¬æÉÄÜŗ¬ÓŠSO42?ŗĶNO3? |
| B£®ŌŚµĪ¼ÓNaOHČÜŅŗĪļÖŹµÄĮæĪŖ0.5ÖĮ0.7molŹ±£¬·¢ÉśµÄĄė×Ó·“Ó¦ĪŖ£ŗAl3+£«4OH-£½[Al(OH)4 ]- |
| C£®ČÜŅŗÖŠµÄŃōĄė×ÓÖ»ÓŠH+”¢Mg2+”¢Al3+ |
| D£®n(H+)”Ćn(NH4+)”Ćn(Mg2+) =2”Ć4”Ć1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĶØČė×ćĮæCO2ŗóµÄČÜŅŗÖŠ£ŗNa+”¢SiO32£”¢CH3COO£”¢CO32£ |
| B£®ĪŽÉ«ČÜŅŗÖŠ£ŗMg2+”¢MnO4£”¢SO42£”¢K+ |
| C£®c(H+)/c(OH£)=1012µÄČÜŅŗÖŠ£ŗNH4+”¢Al3+”¢NO3£”¢Cl£ |
| D£®c(ClO£)=" 1.0" mol/LµÄČÜŅŗ£ŗNa+”¢SO32£”¢S2£”¢SO42£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Na+”¢Mg2+”¢SO42”Ŗ”¢Cl”Ŗ | B£®ClO”Ŗ”¢I”Ŗ”¢NH4+”¢Ba2+ |
| C£®Na+”¢AlO2”Ŗ”¢K+”¢HCO3”Ŗ | D£®Al3+”¢K+”¢SO42”Ŗ”¢NO3”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚ¼ÓČėĀĮ·ŪÄܲśÉśĒāĘųµÄČÜŅŗÖŠ£ŗAl3£«”¢ Fe2+”¢SO42£”¢NO3£ |
| B£®0£®1 mol”¤L-1NaAlO2ČÜŅŗ: H£«”¢Na£«”¢Cl£”¢SO42£ |
| C£®ŌŚŗ¬ÓŠ“óĮæ Fe3£«µÄČÜŅŗÖŠ£ŗNH4+”¢Na£«”¢Cl£”¢SCN£ |
| D£®0.1 mol”¤L-1 CuSO4ČÜŅŗ£ŗH£«”¢K£«”¢NO3£”¢Br- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŃōĄė×Ó | K£«”¢Na£«”¢Fe2£«”¢Ba2£«”¢NH4+ |
| ŅõĄė×Ó | OH£”¢NO3”Ŗ”¢I£”¢HCO3”Ŗ”¢AlO2”Ŗ”¢HSO4”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÄÜŹ¹¼×»ł³Č±ä»ĘµÄČÜŅŗÖŠ£ŗNa+”¢K+”¢SO42£”¢Al3+ |
| B£®pH=2µÄČÜŅŗÖŠ£ŗNa+”¢ClO£”¢NH4+”¢SO42£ |
| C£®Ä³ĶøĆ÷³ĪĒåµÄČÜŅŗÖŠ£ŗNH4£«”¢Cu2+”¢NO3£”¢Cl£ |
| D£®0.1 mol”¤L£1NaHCO3ČÜŅŗÖŠ£ŗK+”¢Ba2+”¢OH£”¢Cl£ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com