
| Ka?c(CH3COOH) |
| Kac(CH3COOH) |
| c(CH3COO-) |
| Ka?c(CH3COOH) |
| a��1.75��10-5 |
| a��1.75��10-5 |
| Kac(CH3COOH) |
| c(CH3COO-) |
| 1.75��10-5��a |
| b |
| 1.75��10-5��a |
| b |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Sn-2e��Sn2+ |
| B������Fe-2e��Fe2+ |
| C������2H2O+O2-2e��4OH- |
| D������Fe-2e��Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ô���ȥ��ˮ����CaCO3+2H+�TCa2++H2O+CO2�� | ||
B����ȩ����������Cu��OH��2��Һ��Ӧ��HCHO+2Cu��OH��2
| ||
C��ʵ������Һ��ͱ��ڴ������������屽��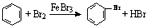 | ||
D����CO2ͨ�뱽������Һ��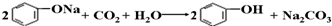 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CF2Cl2 |
| B��C3H6 |
| C��C6H12O6 |
| D��C2H4O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ɳ�����к���CaCO3�������к����� |
| B��������ָ���Խ�ˮ����ˮ��pH��7 |
| C��δ����Ⱦ���������н��µ���ˮһ����������ˮ? |
| D���ļ��������¶Ƚϸߣ�����SO2��NO��ת��Ϊ���������������ף����ļ���ˮ���Ի��ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| NaCN |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
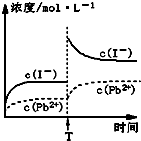 ȡһ������PbI2����������ˮ��ɱ�����Һ����ȡ25.00mLPbI2������Һ���ִ�������R-H��s���������·�Ӧ��Pb2+��aq��+2R-H��s��=R2Pb��s��+2H+��aq������ַ�Ӧ���ˣ�����ƿʢװ��Һ������������ˮϴ�ӳ�������ϴ��Һһ��������ƿ�У�����ָʾ������0.0025mol/LNaOH��Һ�ζ������ﵽ�ζ��յ�ʱ����ȥ����������Һ20.00mL���������ʵ�����ݣ������й�˵����ȷ���ǣ�������
ȡһ������PbI2����������ˮ��ɱ�����Һ����ȡ25.00mLPbI2������Һ���ִ�������R-H��s���������·�Ӧ��Pb2+��aq��+2R-H��s��=R2Pb��s��+2H+��aq������ַ�Ӧ���ˣ�����ƿʢװ��Һ������������ˮϴ�ӳ�������ϴ��Һһ��������ƿ�У�����ָʾ������0.0025mol/LNaOH��Һ�ζ������ﵽ�ζ��յ�ʱ����ȥ����������Һ20.00mL���������ʵ�����ݣ������й�˵����ȷ���ǣ�������| A���Է�̪Ϊָʾ��ʱ���ζ����յ�ʱ��Һ�ɺ�ɫ��Ϊ��ɫ |
| B�����¶Ȳ���ʱ����PbI2������Һ�м�����������ǦŨ��Һ��PbI2��Ksp��С |
| C���¶Ȳ��䣬Tʱ�̣���PbI2������Һ�м�������KIŨ��Һ������Ũ�ȱ仯����ͼ��ʾ |
| D������ʵ���õ�t��PbI2��Ksp=4��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H2Y�ĵ��뷽��ʽΪ��H2Y=2H++Y2- |
| B���ڸ���ʽ����Һ�У�[Na+]��[Y2-]��[HY-]��[OH-]��[H+] |
| C��HY-��ˮ�ⷽ��ʽ��HY-+H2O?H3O++Y2- |
| D���ڸ���ʽ����Һ�У�[Na+]��[HY-]��[OH-]��[H+]��[Y2-] |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com