
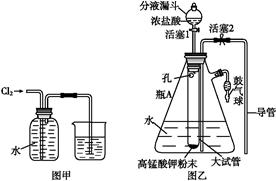
 2HCl+O2��
2HCl+O2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
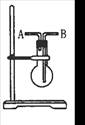
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
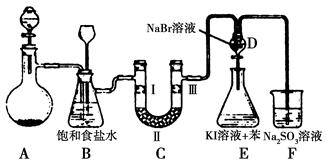
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
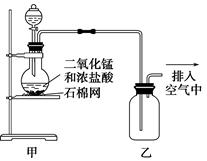
| A����װ��ͼ�����ٴ����������Դ��� |
| B����ƿ�е�MnO2�ɻ���KMnO4 |
| C�����Һ���һʢ�б���ʳ��ˮ���ձ��ɽ���β������ |
| D���ڼ���ƿ�ĵ��ܿڴ���һƬʪ��ĵ���KI��ֽ����֤���Ƿ��������ݳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O��Na2O2���Ԫ����ͬ����CO2��Ӧ�IJ���Ҳ��ͬ |
| B��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
| C��CO��NO��NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ����� |
| D��SiO2���������������NaOH��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
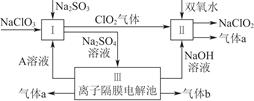
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
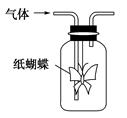
| | A | B | C | D |
| ����Һ | ��̪ | ��ɫʯ�� | �����ظ���� | �ữ��KI������ |
| ͨ������� | NH3 | Cl2 | CH3CH2OH(g) | O3 |
| Ԥ�����ɫ�仯 | ��Ϊ��ɫ | ������ɫ | �����Ա仯 | ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��A��B | B��B��C | C��C��D | D��D��E |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com