
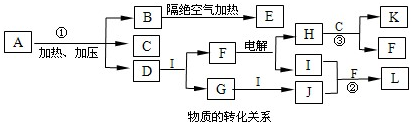
 ”£H2ŹĒŗĻ³É°±µÄŌĮĻ£¬
”£H2ŹĒŗĻ³É°±µÄŌĮĻ£¬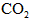 ¹©ŗĻ³ÉÄņĖŲÓĆ”£Čō“Ó³ä·ÖĄūÓĆŌĮĻµÄ½Ē¶Čæ¼ĀĒ£¬Ń”ÓĆ________£ØĢīŠņŗÅ£©ĪļÖŹ×÷ŌĮĻ½ĻŗĆ£®””””
¹©ŗĻ³ÉÄņĖŲÓĆ”£Čō“Ó³ä·ÖĄūÓĆŌĮĻµÄ½Ē¶Čæ¼ĀĒ£¬Ń”ÓĆ________£ØĢīŠņŗÅ£©ĪļÖŹ×÷ŌĮĻ½ĻŗĆ£®””””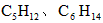 £© C£®
£© C£® ””D£®½¹Ģæ””””
””D£®½¹Ģæ”””” Ė®½āÉś³ÉBiOCl³Įµķ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ””””
Ė®½āÉś³ÉBiOCl³Įµķ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ””””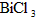 ·Ö½āµÄ·“Ó¦·½³ĢŹ½ĪŖ____________________________________£®””
·Ö½āµÄ·“Ó¦·½³ĢŹ½ĪŖ____________________________________£®””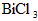 ČÜÓŚÅØNaClČÜŅŗÖŠ»ņČÜÓŚŗ¬ÓŠÉŁĮæŃĪĖįµÄĖ®ÖŠ¶¼µĆµ½³ĪĒåČÜŅŗ£¬ŹŌÓĆĘ½ŗāŅʶÆŌĄķ·ÖĪöæÉÄܵÄŌŅņ____________________________________£®
ČÜÓŚÅØNaClČÜŅŗÖŠ»ņČÜÓŚŗ¬ÓŠÉŁĮæŃĪĖįµÄĖ®ÖŠ¶¼µĆµ½³ĪĒåČÜŅŗ£¬ŹŌÓĆĘ½ŗāŅʶÆŌĄķ·ÖĪöæÉÄܵÄŌŅņ____________________________________£®  ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2-²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

| ·Åµē |
| ³äµē |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
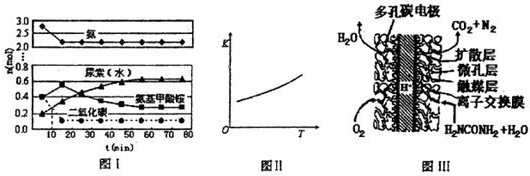
 ėĀ£ØN2H4£©ÓÖ³ĘĮŖ°±£¬¹ć·ŗÓĆÓŚ»š¼żĶĘ½ų¼Į”¢ÓŠ»śŗĻ³É¼°Č¼ĮĻµē³Ų£¬NO2µÄ¶ž¾ŪĢåN2O4ŌņŹĒ»š¼żÖŠ³£ÓƵÄŃõ»Æ¼Į£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ėĀ£ØN2H4£©ÓÖ³ĘĮŖ°±£¬¹ć·ŗÓĆÓŚ»š¼żĶĘ½ų¼Į”¢ÓŠ»śŗĻ³É¼°Č¼ĮĻµē³Ų£¬NO2µÄ¶ž¾ŪĢåN2O4ŌņŹĒ»š¼żÖŠ³£ÓƵÄŃõ»Æ¼Į£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 | 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com