
ÓĆ“æ¼īŗĶĖ«ŃõĖ®»ģŗĻæÉÖĘ×÷ŠĀŠĶŅŗĢåĻ“µÓ¼Į(2Na2CO3”¤3H2O2)£¬Ėü¾ßӊɱ¾śĻū¶¾Č„ÓĶĪŪµÄÄÜĮ¦ĒŅ²»»įĪŪČ¾Ė®Ō“”£
(1) ¼ģŃéÕāÖÖŠĀŠĶĻ“µÓ¼ĮÖŠ½šŹōŃōĄė×ӵIJŁ×÷ŗĶĻÖĻóŹĒ______________________”£
(2) ÕāÖÖĻ“µÓ¼ĮÖŠµÄĖ«ŃõĖ®æÉŅŌ½«·ĻĖ®ÖŠµÄCN- ×Ŗ»ÆĪŖĪŽ¶¾ĪļµÄĶ¬Ź±Éś³ÉNH3£¬Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
(3) Čē¹ūÅäÖĘĻ“µÓ¼ĮµÄĖ®ÖŠŗ¬ÓŠĢśĄė×Ó£¬²»½ö»įĻ÷ČõĻ“µÓ¼ĮµÄČ„ĪŪÄÜĮ¦£¬ÉõÖĮ»įĶźČ«Ź§Č„ɱ¾ś×÷ÓĆ”£ŹŌ·ÖĪöĘäÖŠæÉÄܵÄŌŅņ(Š“³öĘäÖŠŅ»ÖÖ¼“æÉ£¬ÓĆĄė×Ó·½³ĢŹ½ŗĶ¼ņŅŖĪÄ×Ö±ķŹö) ____________________________________________”£
(4) ij»ÆѧѧĻ°Š”×éĪŖĮĖ¶ØŠŌĢ½¾æĢśĄė×Ó¶ŌÕāÖÖŠĀŠĶĻ“µÓ¼ĮµÄ²»Į¼Ó°Ļģ£¬Č”øĆĻ“µÓ¼Į100mL£¬¼ÓČė25gFeCl3¹ĢĢ壬²śÉś“óĮæĪŽÉ«ĪŽĪ¶ĘųĢ壬ÓĆÖüĘųĘæŹÕ¼ÆĘųĢ唣ĒėŃ”ÓĆĻĀĮŠŹŌ¼ĮŗĶŹµŃéÓĆĘ·Ķź³ÉĘųĢå³É·ÖµÄĢ½¾æ¹ż³Ģ:0.10mol”¤L-1NaOHČÜŅŗ”¢8.0mol”¤L-1NaOHČÜŅŗ”¢³ĪĒåŹÆ»ŅĖ®”¢0.10mol”¤L-1KMnO4ČÜŅŗ”¢BaCl2Ļ”ČÜŅŗ”¢Ę·ŗģČÜŅŗ”¢ÕōĮóĖ®”¢Ä¾Ģõ”¢¾Ę¾«µĘ”¢»š²ń”¢Ļ“ĘųĘ攣
¢ŁĢį³ö¼ŁÉč£ŗ¶ŌøĆĘųĢå³É·ÖĢį³öŗĻĄķ¼ŁÉč”£
¼ŁÉč1£ŗĘųĢåŹĒO2”£
¼ŁÉč2£ŗĘųĢåŹĒCO2”£
¼ŁÉč3£ŗĘųĢåŹĒ__________________________”£
¢ŚÉč¼Ę·½°ø:Éč¼ĘŹµŃé·½°øÖ¤Ć÷ÄćµÄ¼ŁÉč£¬ŌŚĻĀ±ķÖŠĶź³ÉŹµŃé²½Öč”¢Ō¤ĘŚĻÖĻóŗĶ½įĀŪ”£
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ½«ĘųĢåŅĄ“ĪĶØČėŹ¢ÓŠ____________________”¢ ””””””””””µÄĻ“ĘųĘæÖŠ£¬²¢________________________________”£ | _________________________________ |
(1)ÓĆ½ą¾»µÄ²¬ĖæÕŗČ”Ļ“µÓ¼ĮŌŚ¾Ę¾«µĘ»šŃęÉĻ×ĘÉÕ£¬»šŃę³Ź»ĘÉ«(ŗĻĄķ¼“æÉ)
(2)H2O2£«CN££«H2O=HCO3”Ŗ£«NH3
(3)2H2O2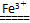 2H2O£«O2”ü£¬ĢśĄė×Ó»į¼ÓĖŁH2O2·Ö½ā£¬Ź¹Ļ“µÓ¼ĮŹ§Č„ɱ¾ś×÷ÓĆ£»2Fe3£«£«3CO32”Ŗ£«3H2O=2Fe(OH)3”ż£«3CO2”ü£¬Fe3£«ÓėCO32”ŖĖ®½āĻą»„“Ł½ų£¬Ź¹Ļ“µÓ¼ĮŹ§Č„Č„ĪŪÄÜĮ¦
2H2O£«O2”ü£¬ĢśĄė×Ó»į¼ÓĖŁH2O2·Ö½ā£¬Ź¹Ļ“µÓ¼ĮŹ§Č„ɱ¾ś×÷ÓĆ£»2Fe3£«£«3CO32”Ŗ£«3H2O=2Fe(OH)3”ż£«3CO2”ü£¬Fe3£«ÓėCO32”ŖĖ®½āĻą»„“Ł½ų£¬Ź¹Ļ“µÓ¼ĮŹ§Č„Č„ĪŪÄÜĮ¦
(4)¢ŁCO2ŗĶO2
¢Ś
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ³ĪĒåŹÆ»ŅĖ® 8.0 mol”¤L£1 NaOHČÜŅŗ””²¢½«“ų»šŠĒµÄŠ”ľĢõ·ÅŌŚ×īŗóŅ»øöĻ“ĘųĘæµÄ³öæŚ“¦ | Čō³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē£¬Ä¾Ģõø“Č¼£¬Ōņ¼ŁÉč1³ÉĮ¢£»Čō³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬Ä¾Ģõø“Č¼£¬Ōņ¼ŁÉč3³ÉĮ¢£»Čō³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬Ä¾Ģõ²»ø“Č¼£¬Ōņ¼ŁÉč2³ÉĮ¢ |


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©ŌŚ400 mL 2 mol”¤L£1µÄH2SO4ČÜŅŗÖŠ£¬ČÜÖŹµÄÖŹĮæŹĒ________”£“ĖČÜŅŗÖŠµÄH+µÄĪļÖŹµÄĮæÅضČĪŖ________£¬SO42£µÄĪļÖŹµÄĮæÅضČĪŖ________”£
£Ø2£©ŌŚ±ź×¼×“æöĻĀ£¬700 L NH3µÄĪļÖŹµÄĮæĪŖ________£¬Č«²æČܽāŌŚ1LĖ®ÖŠ£¬ĖłµĆČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ________”£Čē¹ūøĆ°±Ė®µÄĆܶČĪŖ0.85 g”¤cm£3£¬Ōņ°±Ė®µÄĢå»żĪŖ________£¬NH3µÄĪļÖŹµÄĮæÅضČĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲĻµÖŠ£¬£ĮŹĒŅ»ÖÖÕżŃĪ£¬£ÄµÄ·Ö×ÓĮæ±Č£ĆµÄ·Ö×ÓĮæ“ó16£¬£ÅŹĒĖį£»µ±£ŲĪŽĀŪŹĒĒæ¼ī»¹ŹĒĒæĖįŹ±¶¼ÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ

µ±£ŲŹĒĒæĖįŹ±£¬£Į”¢£Ā”¢£Ć”¢£Ä”¢£Å¾łŗ¬Ķ¬Ņ»ÖÖŌŖĖŲ£»µ±£ŲŹĒĒæĖįŹ±£¬£Į”¢£Ā”¢£Ć”¢£Ä”¢£Å¾łĶ¬Ź±ŗ¬ÓŠĮķŅ»ÖÖŌŖĖŲ”£»Ų“šĻĀĮŠĪŹĢā£ŗ£ØÓĆ»ÆѧŹ½ĢīŠ“£©
£Ø1£©£ĮŹĒ_____________£¬£ŁŹĒ______________£¬£ŚŹĒ____________”£
£Ø2£©µ±£ŲŹĒĒæ¼īŹ±£¬Š“³öBÓėY·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ___________________________”£
£Ø3£©µ±£ŲŹĒĒæĖįŹ±£¬Š“³öCÓėY·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
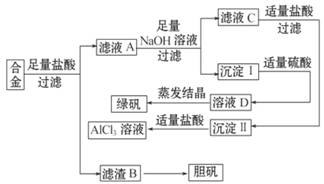 Ä³Ń§Š£»ÆѧŠĖȤŠ”×éĪŖĢ½Ė÷½šŹō»ŲŹÕĪļµÄ×ŪŗĻĄūÓĆ£¬×ØĆÅÉč¼ĘŹµŃéÓĆŗ¬ÓŠĀĮ”¢Ģś”¢ĶµÄŗĻ½šÖĘČ”“æ¾»µÄĀČ»ÆĀĮČÜŅŗ”¢ĀĢ·Æ¾§Ģå(FeSO4”¤7H2O)ŗĶµØ·Æ¾§Ģå£ØCuSO4”¤5H2O)£¬Ę䏵Ńé·½°øČēĻĀ£ŗ
Ä³Ń§Š£»ÆѧŠĖȤŠ”×éĪŖĢ½Ė÷½šŹō»ŲŹÕĪļµÄ×ŪŗĻĄūÓĆ£¬×ØĆÅÉč¼ĘŹµŃéÓĆŗ¬ÓŠĀĮ”¢Ģś”¢ĶµÄŗĻ½šÖĘČ”“æ¾»µÄĀČ»ÆĀĮČÜŅŗ”¢ĀĢ·Æ¾§Ģå(FeSO4”¤7H2O)ŗĶµØ·Æ¾§Ģå£ØCuSO4”¤5H2O)£¬Ę䏵Ńé·½°øČēĻĀ£ŗ
(1)Š”×é³ÉŌ±¾¹ż¼ģ²ā£¬·¢ĻÖÖʵƵÄĀĢ·Æ²»“棬×īæÉÄܵÄŌŅņŹĒ_________________________£»ŅŖĻėÓɳĮµķ¢ń×īÖÕÖʵƓæ¶Č½ĻøßµÄĀĢ·Æ£¬øĽų·½·ØŹĒ__________________________________
(2)Š”×é³ÉŌ±“Ó׏ĮĻÖŠ»ńÖŖH2O2ŹĒŅ»ÖÖĀĢÉ«Ńõ»Æ¼Į£¬ŌŚĀĖŌüBÖŠ¼ÓČėĻ”ĮņĖįŗĶH2O2æÉŅŌŹ¹BČܽā£¬ŌņøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________
(3)ÓŠĶ¬Ń§Ģį³öæɽ«·½°øÖŠ×ī³õČܽāŗĻ½šµÄŃĪĖįøÄÓĆÉÕ¼ī£¬ÖŲŠĀÉč¼Ę·½°ø£¬Ņ²ÄÜÖʵĆÕāČżÖÖĪļÖŹ£¬øĆ·½°ø±ČĒ°Ņ»ÖÖ·½°øĻą¶ŌøüŗĻĄķ£¬ŌŅņŹĒ£ŗ__________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ¶ĢÖÜĘŚŌŖĖŲ¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĘäĒā»ÆĪļÖŠ¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģµÄ»ÆŗĻ¼ŪČēĻĀ±ķ”£ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
| ŌŖ””ĖŲ | ¼× | ŅŅ | ±ū | ¶” | Īģ |
| »ÆŗĻ¼Ū | -4 | +1 | -4 | -2 | -1 |
A£®Ķ¬ÖÜĘŚÖŠ£¬¶”µÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ×īĒæ
B£®ĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ:±ū>¶”
C£®±ūµÄŃõ»ÆĪļÄÜÓėĪģµÄĒā»ÆĪļµÄĖ®ČÜŅŗÄÜ·“Ó¦
D£®Ō×Ó°ė¾¶“󊔣ŗ¼×<±ū£¬ĒŅ¼×ÓŠ¶ąÖÖĘųĢ¬Ēā»ÆĪļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄįĮśŹĒÄæĒ°ŹĄ½ēÉĻ²śĮæ×ī“ó”¢Ó¦ÓĆ·¶Ī§×ī¹ć”¢ŠŌÄÜ±Č½ĻÓÅŅģµÄŅ»ÖÖŗĻ³ÉĻĖĪ¬”£ŅŌXĪŖŌĮĻŗĻ³ÉÄįĮś-66µÄĮ÷³ĢČēĻĀ£ŗ
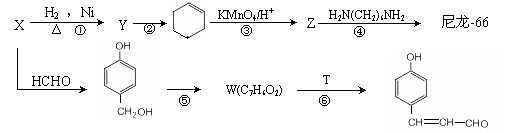
ŅŃÖŖ²æ·ÖŠÅĻ¢ČēĻĀ£ŗ
I£®ZµÄ»ÆѧŹ½ĪŖC6H10O4”£
¢ņ£®XŗĶÅØäåĖ®·“Ó¦²śÉś°×É«³Įµķ”£
¢ó£®Ä³Š©Č©ÓėČ©Ö®¼äÄÜ·¢ÉśōĒČ©ĖõŗĻ·“Ó¦£¬ĄżČē£ŗ
|
|

|
|
|

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©XµÄ»ÆѧĆū³ĘĪŖ______________________”£WµÄ½į¹¹¼ņŹ½ĪŖ______________________”£TµÄ·Ö×ÓŹ½ĪŖ ______________________”£
£Ø2£©·“Ó¦¢ŚµÄĢõ¼žĪŖ____________________________________________________”£
£Ø3£©Š“³ö·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½______________________________________________”£·“Ó¦¢ŻµÄĄąŠĶĪŖ ______________________”£
£Ø4£©GŹĒZÉŁŅ»øöĢ¼Ō×ÓµÄĶ¬ĻµĪļ£¬MŹĒGµÄĶ¬·ÖŅģ¹¹Ģ唣M¼ČÄÜ·¢ÉśŅų¾µ·“Ó¦ŗĶĖ®½ā·“Ó¦”£ÓÖÄÜŗĶĢ¼ĖįĒāÄĘ·“Ó¦²śÉśĘųĢ壬MµÄ½į¹¹¹²ÓŠ___________ÖÖ£¬ĘäÖŠ£¬ŌŚŗĖ“Ź²ÕńĒāĘ×ÉĻÓŠ3øö·åµÄ½į¹¹¼ņŹ½ĪŖ_________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»øö¼ÆĘųĘæÖŠ·ÅÓŠŅ»ĶÅĆŽ»Ø£¬ĻņĘäÖŠµ¹ČėCO2ĘųĢåŹ±ĆŽ»ØČ¼ÉÕĘšĄ“£¬ŌņĆŽ»ØÖŠæÉÄÜ°üÓŠ(””””)
A£®ÉÕ¼ī B£®Ńõ»ÆÄĘ
C£®¹żŃõ»ÆÄĘ D£®Š”ĖÕ“ņ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠŃĪĖį”¢NaClČÜŅŗ”¢NaOHČÜŅŗŗĶŠĀÖĘĀČĖ®£¬æÉÓĆĄ“Ēų±šĖüĆĒµÄŹŌ¼ĮŹĒ(””””)
A£®AgNO3ČÜŅŗ B£®·ÓĢŖČÜŅŗ
C£®×ĻÉ«ŹÆČļČÜŅŗ D£®±„ŗĶŹ³ŃĪĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
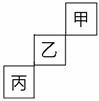 ¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģĪåÖÖĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĘäÖŠ¼×ŅŅ±ū¶¼ĪŖ·Ē½šŹōŌŖĖŲ£¬ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ¹ŲĻµČēĶ¼ĖłŹ¾”£¶””¢ĪģĮ½ÖÖŌŖĖŲŌņĪ»ÓŚĶ¬Ņ»ÖÜĘŚĶ¬Ņ»×壬¶ųĒŅĪģµÄŌ×ÓŠņŹż±Č¶”“ó2”£
¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģĪåÖÖĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĘäÖŠ¼×ŅŅ±ū¶¼ĪŖ·Ē½šŹōŌŖĖŲ£¬ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ¹ŲĻµČēĶ¼ĖłŹ¾”£¶””¢ĪģĮ½ÖÖŌŖĖŲŌņĪ»ÓŚĶ¬Ņ»ÖÜĘŚĶ¬Ņ»×壬¶ųĒŅĪģµÄŌ×ÓŠņŹż±Č¶”“ó2”£
(1)¼×”¢ŅŅ”¢±ūČżÖÖŌŖĖŲµēøŗŠŌÓɓ󵽊”ĪŖ___________________________”£
£ØĢīŌŖĖŲ·ūŗÅ£©
(2)ŅŅµÄĒā»ÆĪļ_______£ØĢī”°ÄÜ”±”¢”°²»ÄÜ”±£©ŠĪ³ÉĒā¼ü£¬ĘäŌŅņĪŖ____________
_________________________________________________________________ӣ
(3)±ūµÄ¼Ūµē×ÓÅŲ¼Ź½ĪŖ_______________________”£
(4) ¹ż¶É½šŹōŌ×ÓæÉŅŌÓėCO·Ö×ÓŠĪ³ÉÅäŗĻĪļ£¬ÅäŗĻĪļ¼Ūµē×Ó×ÜŹż·ūŗĻ18µē×Ó¹ęŌņ”£ČēCræÉŅŌÓėCOŠĪ³ÉCr(CO)6·Ö×Ó£ŗ
¼Ūµē×Ó×ÜŹż£Ø18£©=CrµÄ¼Ūµē×ÓŹż£Ø6£©+COĢį¹©µē×ÓŹż£Ø2”Į6£©
(ĢįŹ¾£ŗĆæøöCOÅäĢåĢį¹©2øöµē×Ó)
 ¶””¢ĪģĮ½ÖÖŌ×Ó¶¼ÄÜÓėCOŠĪ³ÉÅäŗĻĪļ£¬Ęä»ÆѧŹ½·Ö±šĪŖ__________________”¢_________________”£
¶””¢ĪģĮ½ÖÖŌ×Ó¶¼ÄÜÓėCOŠĪ³ÉÅäŗĻĪļ£¬Ęä»ÆѧŹ½·Ö±šĪŖ__________________”¢_________________”£
(5) ¶”µÄ¾§°ūČēĶ¼ĖłŹ¾£¬ŅŃÖŖ¶”Ļą¶ŌŌ×ÓÖŹĮæĪŖM£¬¾§ĢåĆÜ¶Č¦Ń g”¤cm£3£¬°¢·üŁ¤µĀĀŽ³£ŹżĪŖNA£¬
¶”ŌŖĖŲµÄŌ×Ó°ė¾¶ĪŖ__________________cm”£
£ØÓĆM”¢¦Ń”¢NA±ķŹ¾£¬²»ÓĆ»Æ¼ņ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com