
·ÖĪö £Ø1£©¢ŁĀČ»ÆĒāĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė0.1mol•L-1µÄŃĪĖįĒāĄė×ÓÅضČĪŖ0.1mol•L-1£»¢ŚĮņĖįĪŖĒæµē½āÖŹ0.2mol•L-1µÄĮņĖį£¬ĒāĄė×ÓÅضČĪŖ””0.4mol•L-1£»””¢Ū“×ĖįĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ“×ĖįĒāĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»¢ÜŅ»Ė®ŗĻ°±ĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ°±Ė®ĒāŃõøłĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»””¢ŻĒāŃõ»ÆÄĘĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄNaOHČÜŅŗÖŠĒāŃõøłĄė×ÓÅضČ0.1mol•L-1””¢ŽĒāŃõ»Æ±µĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄBa£ØOH£©2ČÜŅŗ£¬ĒāŃõøłĄė×ÓÅضČĪŖ0.2mol•L-1£»
£Ø2£©µē½āÖŹŹĒÖø£ŗŌŚĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄܹ»µ¼µēµÄ»ÆŗĻĪļ£®µē½āÖŹĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄܹ»µ¼µē£¬ŹĒŅņµē½āÖŹ×ŌÉķæÉŅŌĄė½ā³É×ŌÓÉŅĘ¶ÆµÄĄė×Ó£»
·Ēµē½āÖŹŹĒÖø£ŗŌŚĖ®ČÜŅŗĄļŗĶČŪȌדĢ¬ĻĀ¶¼²»µ¼µēµÄ»ÆŗĻĪļ£»µ„ÖŹ”¢»ģŗĻĪļ¼Č²»ŹĒµē½āÖŹŅ²²»ŹĒ·Ēµē½āÖŹ£»
Ēæµē½āÖŹŹĒŌŚĖ®ČÜŅŗÖŠ»ņČŪȌדĢ¬ĻĀÄÜĶźČ«µēĄėµÄµē½āÖŹ£¬°üĄØĒæĖį”¢Ēæ¼ī”¢»īĘĆ½šŹōŃõ»ÆĪļŗĶ“ó²æ·ÖŃĪ£»
£Ø3£©¼ÓĖ®Ļ”ŹĶČõĖįŹ±£¬»į“Ł½ųČõĖįµÄµēĄė£»ŅĄ¾ŻÖŠŗĶ·“Ó¦ÖŠĻūŗĵÄĒāĄė×ÓµÄĪļÖŹµÄĮæµČÓŚĒāŃõøłĄė×ÓµÄĪļÖŹµÄĮæ½ā“š£»
£Ø4£©Éč³öĖįČÜŅŗµÄpHĪŖa£¬¼īČÜŅŗµÄpHĪŖb£¬øł¾ŻøĆĪĀ¶ČŅŌ¼°Ģå»ż¹ŲĻµĮŠŹ½¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©£©¢ŁĀČ»ÆĒāĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė0.1mol•L-1µÄŃĪĖįĒāĄė×ÓÅضČĪŖ0.1mol•L-1£»¢ŚĮņĖįĪŖĒæµē½āÖŹ0.2mol•L-1µÄĮņĖį£¬ĒāĄė×ÓÅضČĪŖ””0.4mol•L-1£»””¢Ū“×ĖįĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ“×ĖįĒāĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»¢ÜŅ»Ė®ŗĻ°±ĪŖČõµē½āÖŹ£¬²æ·ÖµēĄė£¬0.1mol•L-1µÄ°±Ė®ĒāŃõøłĄė×ÓÅØ¶ČŠ”ÓŚ0.1mol•L-1£»””¢ŻĒāŃõ»ÆÄĘĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄNaOHČÜŅŗÖŠĒāŃõøłĄė×ÓÅضČ0.1mol•L-1””¢ŽĒāŃõ»Æ±µĪŖĒæµē½āÖŹ£¬ĶźČ«µēĄė£¬0.1mol•L-1µÄBa£ØOH£©2ČÜŅŗ£¬ĒāŃõøłĄė×ÓÅضČĪŖ0.2mol•L-1£»
ĒāĄė×ÓÅضČŌ½“ó£¬pHŌ½Š”£¬ĒāŃõøłĄė×ÓÅضČŌ½“ó£¬pHŌ½“ó£¬ĖłŅŌpHÓÉŠ”µ½“óµÄĖ³Šņ£ŗ¢Ś¢Ł¢Ū¢Ü¢Ż¢Ž£»
¹Ź“š°øĪŖ£ŗ¢Ś¢Ł¢Ū¢Ü¢Ż¢Ž£»
£Ø2£©¢ŁNaClŹĒŃĪ£¬ČŪȌדĢ¬»ņÕßČÜÓŚĖ®Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»””¢ŚBaSO4ŹĒŃĪČŪȌדĢ¬Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»
¢ŪH2SO4ŹĒĒæĖį£¬ČÜÓŚĖ®Äܹ»ĶźČ«µēĄė£¬ŹōÓŚĒæµē½āÖŹ£»¢ÜKOHŹĒĒæ¼ī£¬ČÜÓŚĖ®»ņÕßČŪȌדĢ¬ÄÜĶźČ«µēĄėŹōÓŚĒæµē½āÖŹ£»
¢ŻŹÆÄ«ŹĒµ„ÖŹ¼Č²»ŹĒµē½āÖŹ£¬Ņ²²»ŹĒ·Ēµē½āÖŹ£»””¢ŽH2CO3ŹĒČõĖįĖ®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£»
¢ßCH3COOH””ŹĒČõĖįĖ®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£»¢ąNH3•H2O ŹĒČõ¼ī£¬Ė®ČÜŅŗÖŠÄܲæ·ÖµēĄė£¬ŹōÓŚČõµē½āÖŹ£» ¢įÕįĢĒŌŚĖ®ČÜŅŗŗĶČŪȌדĢ¬¶¼²»µ¼µē£¬ŹōÓŚ·Ēµē½āÖŹ£»””¢ā¾Ę¾«ŌŚĖ®ČÜŅŗŗĶČŪȌדĢ¬¶¼²»µ¼µē£¬ŹōÓŚ·Ēµē½āÖŹ£»””””£Ø11£©SO2””±¾Éķ²»ÄܵēĄė£¬ŹōÓŚ·Ēµē½āÖŹ£»£Ø12£©Cu µ„ÖŹ£¬¼Č²»ŹĒµē½āÖŹŅ²²»ŹĒ·Ēµē½āÖŹ£»
£Ø13£©Cl2 ŹĒµ„ÖŹ¼Č²»ŹĒµē½āÖŹ£¬Ņ²²»ŹĒ·Ēµē½āÖŹ£»£Ø14£©CO2£¬±¾Éķ²»ÄܵēĄė£¬ŹōÓŚ·Ēµē½āÖŹ£»
ŹōÓŚĒæµē½āÖŹµÄÓŠ£ŗ¢Ł¢Ś¢Ū¢Ü£»ÖŠŹōÓŚČõµē½āÖŹµÄÓŠ£ŗ¢Ž¢ß¢ą£»ĘäÖŠŹōÓŚ·Ēµē½āÖŹµÄÓŠ¢į¢ā£Ø11£©£Ø14£©£»
¹Ź“š°øĪŖ£ŗ¢Ł¢Ś¢Ū¢Ü£»¢Ž¢ß¢ą£»¢į¢ā£Ø11£©£Ø14£©£»
£Ø3£©Ä³Ņ»ŌŖČõĖįČÜŅŗ£ØA£©Óė¶žŌŖĒæĖį£ØB£©µÄpHĻąµČ£¬ŌņČõĖįÓŠ“ó²æ·ÖĪŖµēĄė£¬¼ÓĖ®Ļ”ŹĶŹ±£¬Äܹ»“Ł½ųøü¶ąµÄČõĖįµēĄė£¬ĖłŅŌ¼ÓĖ®Ļ”ŹĶŗó£¬ĒāĄė×ÓÅØ¶Č“óÓŚ¶žŌŖĒæĖįÖŠĒāĄė×ÓÅØ¶Č£¬ĖłŅŌpHA£¼pHB£¬
ÉĻŹöĻ”ŹĶČÜŅŗÖŠČõĖįµÄĒāĄė×ÓµÄĪļÖŹµÄĮæÅØ¶Č“óÓŚĒæĖį£¬ĖłŅŌµČĢå»żŗ¬ÓŠµÄĒāĄė×ÓµÄĪļÖŹµÄĮæ¶ą£¬ÖŠŗĶµČÅØ¶ČµČĢå»żµÄNaOHČÜŅŗ£¬ÓƵÄĢå»żÉŁ£»
¹Ź“š°øĪŖ£ŗ£¼£»£¼£»
£Ø4£©ÉčĒæĖįČÜŅŗµÄpHĪŖa£¬Ģå»żĪŖV£¬pH=-lgc£ØH+£©£¬ČÜŅŗÖŠĒāĄė×ÓÅضČĪŖ£ŗ10-amol/L£»¼īČÜŅŗµÄpHĪŖb£¬Ģå»żĪŖ10V£¬ČÜŅŗÖŠĒāŃõøłĄė×ÓµÄÅضČĪŖ£ŗc£ØOH-£©=10-£Ø14-b£©mol/L£¬»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬ŌņĀś×ćČÜŅŗÖŠĒāĄė×ÓµÄĪļÖŹµÄĮæ“óÓŚĒāŃõøłĄė×ÓµÄĪļÖŹµÄĮ棬¼“10-amol/L”ĮVL=10-£Ø14-b£©mol/L”Į10VL£¬
½āµĆ£ŗ-a=b-13£¬a+b=13£¬¼“pHĖį+pH¼ī=13£¬
¹Ź“š°øĪŖ£ŗpHĖį+pH¼ī=13£»
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×ÓÅØ¶Č“óŠ”µÄ±Č½Ļ£¬µē½āÖŹ”¢Ēæµē½āÖŹ”¢·Ēµē½āÖŹµÄÅŠ¶Ļ£¬ČÜŅŗĖį¼īŠŌÓėpHÖµµÄĻą¹Ų¼ĘĖć£¬ĢāÄæ×ŪŗĻŠŌĒ棬Éč¼ĘÄŚČŻ¶ą£¬ÄѶČÖŠµČ£¬½āĢāŹ±×¢Ņā°ŃĪÕµē½āÖŹĒæČõµÄÅŠ¶ĻŅĄ¾Ż£¬×¢ŅāČõµē½āÖŹµēĄėµÄĢŲµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C3H6£¬C4H8 | B£® | C3H6£¬C4H6O | C£® | C2H6£¬C3H6O2 | D£® | CH4O£¬C3H4O2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
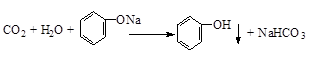 £®
£®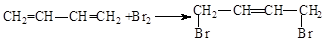 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČĖŌģĆ« | B£® | Õ³½ŗĻĖĪ¬ | C£® | Ć«µÓĀŚ | D£® | Āé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ¹²¼Ū¼ü | H-H | O=O | H-O |
| ¼üÄÜ/kJ•mol-1 | 436 | 498 | X |
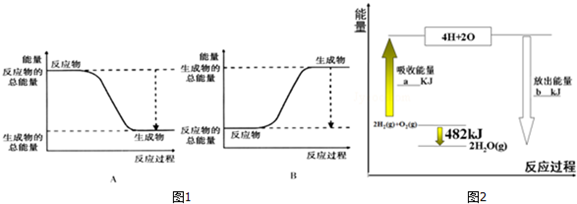
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ÓŠ»śĪļAŹĒŅ»ÖÖŗ¬äåµÄõ„£¬·Ö×ÓŹ½ĪŖC6H9O2Br£¬ŅŃÖŖÓŠČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ŗ
ÓŠ»śĪļAŹĒŅ»ÖÖŗ¬äåµÄõ„£¬·Ö×ÓŹ½ĪŖC6H9O2Br£¬ŅŃÖŖÓŠČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ŗ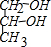 »ņHOCH2CH2CH2OH£®
»ņHOCH2CH2CH2OH£® £ØR±ķŹ¾Ģž»ł£©µÄÓŠ2ÖÖ£®
£ØR±ķŹ¾Ģž»ł£©µÄÓŠ2ÖÖ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹģŹÆ»ŅÓėĮņĖįļ§»ģŗĶŃŠÄ„ NH4++OH-ØTNH3”ü+H2O | |
| B£® | ĻņKOHČÜŅŗÖŠĶØČėÉŁĮæµÄSO2 ĘųĢå SO2+OH-ØTHSO3- | |
| C£® | ĶʬĶ¶ČėĻ”ĻõĖįÖŠ 3Cu+8H++2 NO3-ØT3Cu2++2NO”ü+4H2O | |
| D£® | ĀČĘųĶØČėĖ®ÖŠ Cl2+H2OØT2 H++Cl-+ClO- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 C
C E
E
 £»
£» £»
£» £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | K+£¬Cu2+£¬Cl-£¬SO42- | B£® | NO3-£¬Mg2+£¬S2-£¬SO42- | ||
| C£® | HCO3-£¬I-£¬Ca2+£¬Cl- | D£® | K+£¬Ca2+£¬CO32-£¬Cl- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com