

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��3mol/LHCl��Һ | B��4mol/LHNO3��Һ |
| C��8mol/LNaOH��Һ | D��18mol/LH2SO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ��Һ | ��������� | ����� |
| �ٱ���ʯ��ˮ | ͨ����CO2 | |
| ��AlCl3��Һ | ͨ����NH3 | |
��MgCl2��AlCl3����� Һ Һ | ��μ�NaOH��Һ������ | |
| ��AlCl3��Һ | ��μ�NaOH��Һ������ | |
| �ݺ�����HCl��AlCl3��Һ | ��μ�NaOH��Һ������ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������AI��ʣ�� | B��c(Na+)=c(AlO-2) |
| C��������Һ�ʼ��� | D��Na�����ʵ�����0.6mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
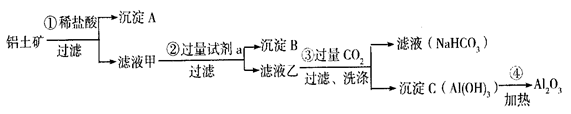
 A��B�ijɷֱַ��� �� ������� �е��Լ�a�� ��
A��B�ijɷֱַ��� �� ������� �е��Լ�a�� �� �д���Fe3���IJ���������__________ _��
�д���Fe3���IJ���������__________ _���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �������ȼ��ڸ����·����ķ�Ӧ�����ȷ�Ӧ���磺2Al��
�������ȼ��ڸ����·����ķ�Ӧ�����ȷ�Ӧ���磺2Al�� Fe2O32Fe��Al2O3����Ӧ����ʱ�ų��������ȡ���ϸ�Ķ���
Fe2O32Fe��Al2O3����Ӧ����ʱ�ų��������ȡ���ϸ�Ķ��� ����Ϣ�ش��������⣺
����Ϣ�ش��������⣺ ��6��θ��ƽ������θ�ᣨHCl������ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ���������������������������������� ��
��6��θ��ƽ������θ�ᣨHCl������ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ���������������������������������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��þ���������������ɺϽ�þ���Ͻ��ǿ�ȸߣ���е���ܺá��С���������������������Ȼ���е�þԪ����Ҫ�����ں�ˮ�С���ˮ��þ���ܴ���ԼΪ1.8��1015 t���Ӻ�ˮ�У���Ҫ����Na+��Cl����Mg2+�����ӣ���ȡþ�Ĺ�������ͼ���£�
��þ���������������ɺϽ�þ���Ͻ��ǿ�ȸߣ���е���ܺá��С���������������������Ȼ���е�þԪ����Ҫ�����ں�ˮ�С���ˮ��þ���ܴ���ԼΪ1.8��1015 t���Ӻ�ˮ�У���Ҫ����Na+��Cl����Mg2+�����ӣ���ȡþ�Ĺ�������ͼ���£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �����ռ���CO2��Ȼ����10mLŨNaO
�����ռ���CO2��Ȼ����10mLŨNaO H��Һ��Ѹ���ܷ������ڣ����Թ۲쵽������ͻȻ����ˣ�ԭ���� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ�����ӷ���ʽΪ �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
H��Һ��Ѹ���ܷ������ڣ����Թ۲쵽������ͻȻ����ˣ�ԭ���� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ�����ӷ���ʽΪ �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ� �ߣߣߣߣߣߣߣߣߣߣߡ�
�ߣߣߣߣߣߣߣߣߣߣߡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����������Ϊ80 mL | B��a��ȡֵ��ΧΪ0 < a��50 |
| C��n (Mg2+) < 0. 025 mol | D����aֵΪ30ʱ��bֵΪ0.01 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com