
ʵ���һ�ȡ�����кܶ���;����������3�ַ����ǣ�
����һ��������ͨ�����ȵ�����ͭ��ĩ���õ������ĵ�����ͭ��
��������������ͨ�����ȵ�ͭ���õ��ϴ����ĵ���������ͭ��ĩ��
�����������������ƣ�NaNO2�����Ȼ�淋Ļ����Һ���ȣ���Ԫ��ȫ��ת��Ϊ������
����ʵ��ʱ��ѡ���ʵ����������ͼ��ʾ��ʡ�Լг�װ�������װ�ã���
��1������һ���Ƶ�������İ���������Ũ��ˮ�μӵ���ʯ���еõ����˷�Ӧ�ķ���װ�����ѡ��_____________________��ѡ��װ�ñ�ţ�����д����ʯ���ڴ˷�Ӧ�е��������ã�__________________________________,___________________________________________.
��2����������Ϊ��֤���õ��������ܴ���������ʹ��ͭ�⣬��������װ��_________________
��ѡ��װ�ñ�ţ��м���_________________________�Գ�ȥ�����������塣
��3�����������Ƶ����Ļ�ѧ����ʽΪ��_____________________________________________��
��4����ɫ��ѧ��ָ�������Ӧ�û�ѧ��ƷʱӦ��Ч���ã���ÿ�������ԭ�ϣ���������ͱ���ʹ���ж��ĺ�Σ�յ��Լ����ܼ�������ȡ������3�ַ����У�����ʹ�÷������ͷ������뵥��ʹ�÷�������ȣ����кܶ���Խ�ԣ������ɫ��ѧ�ĽǶȽ������ۣ�_____________
______________________________________________________________________________.
��5�� 1892�꣬Ӣ����ѧ��������Rayleigh�����֣����÷������õ��ĵ�������ͬ�����±ȷ������õ��ĵ����ܶ�����ƫ��5�����ҡ�������ʵ�����������������ҵ�������ȫ���
����Ͳ�����һ�����ԭ��_______________________________________________.
��1��D�� CaO��ˮ��Ӧ���������ܼ���CaO��ˮ��Ӧ���ȣ������˰������ܽ�ȡ�
��2��E ��ʯ��
��3��NaNO2+NH4Cl NaCl+N2��+2H2O��
NaCl+N2��+2H2O��
��4��Cu��CuO����ѭ��ʹ�ã���ʡ�Լ�������ʹ���ж�����NaNO2�������Ⱦ��
��5���������Ƶĵ�������ϡ��������ܶȱȵ����ܶȴ����Ե��µ����ܶ�ƫ��
���������������1����CaO��Ũ��ˮ��Ӧ��Ӧѡ��Һ�����͵ķ���װ�ã���D��Ũ��ˮ�����¸���ʯ�ҷ�Ӧ���ɰ������������ƣ���ѧ����ʽΪCaO+NH3?H2O=NH3��+Ca��OH��2����Ϊ��ˮ���ȶ��ӷ�����ʯ�Һ�ˮ��Ӧ������ʯ�ҵĹ����У���ʯ�ҵ����������ǣ�һ�������İ�ˮ��Һ�е�ˮ����һ����ų���������ʹ��ˮ�ӷ����ֽ���õ�������
��2�������г��˵����������⣬�����ж�����̼��ˮ�����ȣ�Ϊ��֤���ð��������ܴ�����Ҫ��ȥ�����ж����ˮ������������̼�����ʣ�����ͨ�����Ը����-��ʯ�ң��ȿ������ն�����̼��������ˮ��
��3��������Ϣ��Ӧ�����������ƣ�NaNO2�����Ȼ�泥���Ӧ�����Ǽ��ȣ���Ԫ��ȫ��ת��Ϊ��������Ӧǰ��Ԫ�ص�����䣬��������ﻹ���Ȼ��ƺ�ˮ����˷�Ӧ�ķ���ʽΪ��
NaNO2+NH4Cl NaCl+N2��+2H2O��
NaCl+N2��+2H2O��
��4����������Ϣ��֪��NH3��CuO����Cu��N2��������Cu����CuO����Cu��CuO����ѭ��ʹ�ã�������ʹ���ж����������ƣ�����һ�Ͷ�����ʹ���ж����ʶ������Ⱦ���������ʹ�÷���һ�ͷ������뵥��ʹ�÷�������ȵ��ŵ��У�ͭ������ͭ��ѭ��ʹ�ã���ʡ�Լ��������ʹ���ж����ʶ������Ⱦ����
��5��������ͨ�����ȵ�ͭ���õ��ϴ����ĵ���������ͭ��ĩֻ�dz�ȥ�����������л�����ϡ����������ʣ�����ϡ��������ܶȱȵ����ܶȴ����Ե��µ����ܶ�����ƫ��5�����ң�
���㣺�������Ʊ�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(12��)SnSO4��һ����Ҫ�������Σ��ڹ�ҵ���������Ź㷺��Ӧ�á����Ʊ�·�����£�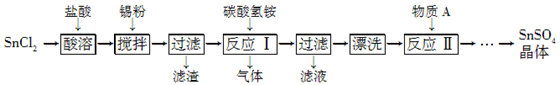
��֪�������������£���Һ�е�Sn2���ɱ������е�����������Sn4���� SnCl2��ˮ�����ɼ�ʽ�Ȼ�����[Sn(OH)Cl]��
��1�� д������A�����ƣ�________��
��2�� SnCl2�����������ˮ�ܽ��ԭ����____________________(�û�ѧ����ʽ��ʾ)��
��3�� ���۵������dz�ȥ����ʱ����������Sn4������д������Sn4�������ӷ���ʽ��______________________________��
��4�� ��Ӧ�����ɵij���ΪSnO��д���÷�Ӧ�Ļ�ѧ����ʽ��____���÷�Ӧ���¶���Ҫ������75 �����ҵ�ԭ����____��
��5�� ʵ�����С�Ưϴ��������ʵ�����������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣���ѧʵ���ǿ�ѧ̽���Ļ�������ش��й�ʵ�����⣺
��1��������ĸ�ʵ��װ������������������ȱ�ݣ�������ȫ��ȷ���� ��
��2��Ҫ��������Bװ�ð����Ѽ����IJ����� ���Թ��Ѽ�����
��3��ClO2��һ�ְ�ȫ����Ч�����ס�ǿ��ɱ���������������ұ���
| ɫ̬ | ���ڼ� | ����1Kpa�����Ȼ����� | |
| �������� | -59-11����ɫҺ�� | �����������κ������� | ��ը |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼��ͭ��һ�ֻ���ԭ�ϣ���ѧʽ��mCu(OH)2��nCuCO3��ʾ��ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�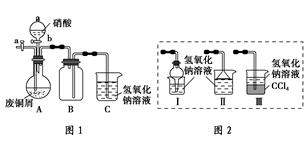
��.��ͭм������ͭ
����1����ͭм�ڿ����г�����գ�������������ϡ���
����2����ͼ1(�г�������ʡ��)����Ũ���Ỻ���ӵ���ͭм��(��ͭм����)����ַ�Ӧ����ˣ��õ�����ͭ��Һ��
����3��������2��Ũ���ỻ��ϡ���ᣬ�������䡣
��.��ʽ̼��ͭ���Ʊ�
������Թ��м���̼������Һ������ͭ��Һ
��ˮԡ������70 ������
����0.4 mol��L��1��NaOH��Һ����pH��8.5�������á�����
������ˮϴ�ӡ���ɣ��õ���ʽ̼��ͭ��Ʒ
��ش��������⣺
(1)������1ʵ�飬����ѡ�������������________(�����)��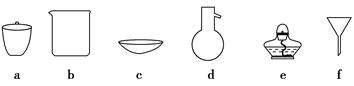
(2)ͼ2���ֱܷ����ͼ1��B��Cװ�õ���________(��װ�����)��
(3)��֪��NO��NO2��2NaOH===2NaNO2��H2O��2NO2��2NaOH===NaNO3��NaNO2��H2O��NO���ܵ�����NaOH��Һ��Ӧ��ʵ�����ʱ����β�������ʹװ���е��ж����屻NaOH��Һ��ȫ���գ�__________________________��
(4)�������ϴ�ӵ�Ŀ����______________________________________��
(5)����۹��˺����Һ�к���CO32��������CO32���ķ�����_________________________________________________________��
(6)�ⶨ��ʽ̼��ͭ��ɵķ�����Ҫ�����֣�
����1�����շ���ȡ34.6 g������mCu(OH)2��nCuCO3����Ӳ���Թ������գ��������������ͨ��������Ũ���ᡢ�����ļ�ʯ���У���ȫ���պ�Ũ���Ά��1.8 g����ʯ�Ҿ���8.8 g��
����2����ԭ�����������м�ǿ�ȣ��ⷴӦǰ������������
�������������������ʽ̼��ͭ�Ļ�ѧʽ_____________________��
����ƽ��ѧ����ʽ��mCu(OH)2��nCuCO3��________H2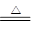 ________Cu��________CO2����________H2O
________Cu��________CO2����________H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼���һ�ֽϳ�ʹ�õĻ��ʣ����ڳ������ֽ⡣ij��ѧ��ȤС���̼淋ijɷִ������ʣ�����������̽����
������ʵ�顿������Һ�е����������ӡ�
ȡ������������Թ��У��������ᣬ�����ɵ�����ͨ�����ʯ��ˮ�У��а�ɫ�������ɡ�����ȡ����̼立����Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�������ɵ����壬ʯ����ֽ����ɫ��
��1������ʵ�������Ʋ�̼��������������ӿ�������������������������
��2����ʵ������̼�������NaOH��Һ���ȷ�Ӧ�����ӷ���ʽ���������� ����
������ʵ�顿�ⶨ̼���CԪ�غ�NԪ�������ȡ�
����ȤС��ȷ��ȡag̼泥�����ʹ֮�ֽ⣬���Ѳ���ͨ���ʯ���У�����ͼ��ʾ��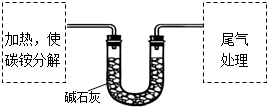
��1��̼粒���Ӧ���������������н��м��ȡ�
| A���Թ� | B�������� | C����ƿ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о�С����ʵ��������ͭ��(������5%����)��ȡ����(CuSO4��5H2O)�������䴿�ȣ���ѡ�Լ���3 mol��L��1H2SO4��Һ��Ũ���ᡢ3%H2O2��Һ��0.2 mol��L��1NaOH��Һ��20% KSCN��Һ��BaCl2��Һ���й������ܽ�����±�(��λg/100 g H2O)��
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| CuSO4 | 14.3 | 20.7 | 28.5 | 40.0 | 55.0 | 75.4 |
| Cu(NO3)2 | 81.8 | 124.8 | 163.1 | 181.8 | 207.8 | 247.3 |
| ʵ�鲽�� | Ԥ������ |
| ����1����������ͭ�������ձ��У�__________________ | _______________________ |
| ����2���ܽ⡣������1�õ���ͭ�������ձ��У� __________________________ | _______________________ |
| ����3���ᾧ��������2������Һ����Ũ����Һ���о�Ĥ���֣���ȴ�����£����˵ôֲ�Ʒ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��13�֣�ij��ѧ��ȤС���ͬѧ����ͼ��ʾʵ��װ�ý���ʵ���о�(ͼ��a��b��c��ʾֹˮ��)������䷽���������ƻ����ۡ�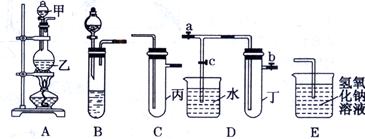
��1��ʵ���ҽ�B��C��E��������Ũ����� Ϊԭ�Ͽ���ȡCl2��
��2������ʵ���ҳ��÷�����ȡ��������A��C��E�������ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ��������£�������������±���
| ʵ�� ��� | ʵ����� | ���� |
| �� | ����ˮ����Ʒ����Һ | �� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ�����ô�п��ϡ���ᷴӦ��ȡ����������������ԭ����ͭ���ⶨͭ�����ԭ��������ʵ��װ�����£�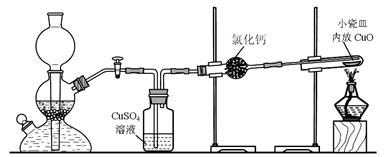
��1�������շ������д�п��ϡ���ᷴӦ���ڻ������ɲ�ȡ�ļӿ����ʵĴ�ʩ�Ǣ��ʵ����������Ũ�ȣ���_______________________��
��2���ô��Ȳ��ߵ�п(��п)��ȡ��������Ӧ���ʿ죬���Ƶõ�������H2S�Ȼ�ԭ�����ʶ������ŵ���ζ��CuSO4ϴ��ƿ�й۲쵽��������_______________���Ƿ���Խ�CuSO4��Һ����Ũ����_________(���ǡ���) ��������__________________________��
��3����ͬѧ���ִ�п��ϡ���ᷴӦһ��ʱ���п�������ڣ������ռ��������ú�ɫ���壬��֤��ɫ���庬��Ԫ�صļ�ѧ����_________________________________________��
��4��ʵ���еõ��������У�С���������mg��С�������Ʒ������n g����Ӧ��С����ӹ��������w g����֪�������ԭ������Ϊ16����ͭ�����ԭ��������____________(�ú�m��n��w�Ĵ���ʽ��ʾ)��
��5��ij��ʵ���вⶨ�������ƫ���ܵ�ԭ����________(ѡ����)��
a��δ�����ȴ��ֹͣͨ���� b������ͭ��Ʒ�к����Ȼ������
c����Ӧ�������������Cu2O d������������������ˮ��
��6���������շ�����ҩƷ�����϶࣬������������װ��һ�������շ��������ܵļ���װ�ã���ѡ��____________(ѡ����) (֧����������Ƥ������������)��
(a) (b) (c) (d) (e) (f)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
| A������100 mL 1��0 mol/L CuSO4��Һ���ɽ�25 g CuSO4��5H20����100 mL����ˮ�� |
| B�����������ͷ����ˮ�У�һ��ʱ���ȡ������Һ���Թ��У���AgNO3��Һ��ϡ�����NaNO2��Һ�������ְ�ɫ������˵��������Ԫ�� |
| C����ֽ�ϲ���������ijЩ����ʱ��Ϊ�˿���ɫ�ߣ�ֻ����ɫ���ӵ����ʲſ�����ֽ������ |
| D����ѹ����ʱ������ƿ��Һ��߶Ƚ��ﵽ֧�ܿ�ʱ��Ӧ�ε�����ƿ�ϵ���Ƥ�ܣ���������ƿ֧�ܿڵ�����Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com