
 ______���ƫ�ߡ��������䡱��ƫ�͡�����
______���ƫ�ߡ��������䡱��ƫ�͡�����| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ�1�У��μӹ���lmol/L�Ȼ�����Һ�� | �������ֻ��ǣ�����Һ�в�����SO32-�� �����ֻ��ǣ�����Һ�п��ܺ���SO32-�� |
| ����2�������ֻ��ǣ�����һ��ʱ����ϲ���Һ�����Թ�2�С����Թ�1�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ���ټ��� �� | |
| ����3�� | |
| ʵ����� | Ԥ����������� |
| ����2�� 2mol/L���ᡣ��1�֣� | ���������������壬��֤����Һ�д���SO32-�� �������壬����SO32-����2�֣� |
| ����3�����Թ�2�м������2mol/L���ᣬ�ٵ���2��Ʒ�졣 �����Թ�2�м������lmol/L����������Һ����3�֣� | ��ɫ��ȥ�������HSO3-����ɫ����ȥ������HSO3-�� ���ֻ��ǣ������HSO3-�������ֻ��ǣ�����HSO3-����2�֣� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 mL������һλС������
mL������һλС������ L���ֹ�����Ͳ����ѡ�õ���Ͳ�� ��������ţ�
L���ֹ�����Ͳ����ѡ�õ���Ͳ�� ��������ţ� �͡����䡱����
�͡����䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
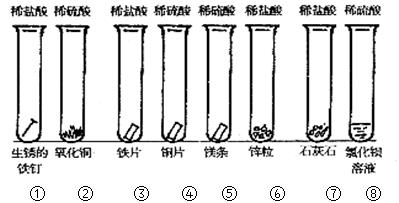
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
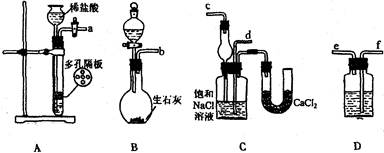
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
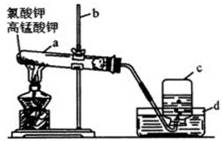
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ� | �ζ����� | ������Һ���/ml | ��NaOH��Һ���������ml�� | |
| �ζ�ǰ/ml | �ζ���/ml | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.30 | 22.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
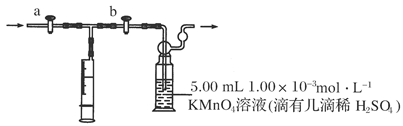
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
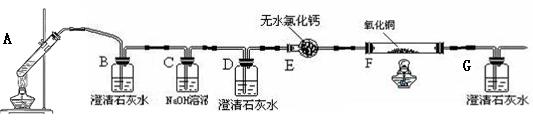 ��1��װ��C�������� _______��װ��E�������� ��
��1��װ��C�������� _______��װ��E�������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com