
��10�֣���ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B�������ʵ�����ݰ�Ҫ����գ�
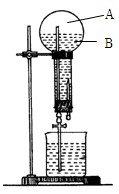
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B�������ʵ�����ݰ�Ҫ����գ�
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�����ʡɳ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣���ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B �������ʵ�����ݰ�Ҫ����գ�
�������ʵ�����ݰ�Ҫ����գ�
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B�������ʵ�����ݰ�Ҫ����գ�
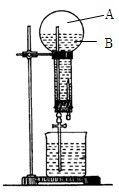
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B�������ʵ�����ݰ�Ҫ����գ�

��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com